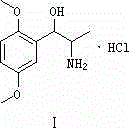
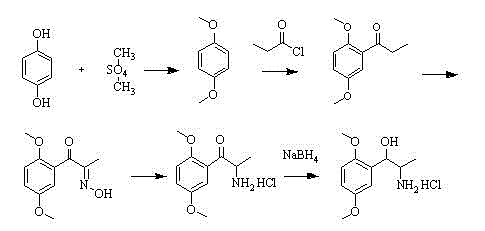
Method for preparing methoxamine hydrochloride
A technology of methoxamine hydrochloride and a synthesis process, which is applied in the field of preparation of cardiovascular drug methoxamine hydrochloride, can solve the problems of difficult gas flow control, unstable yield, long reaction steps, etc., and achieves fewer reaction steps, simple operation, The effect of mild operating conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
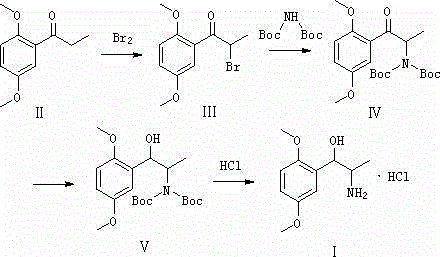
[0022] Embodiment 1: the synthesis of compound III
[0023] 1g (0.005mol) p-methoxy bromopropiophenone (compound II) was dissolved in 15ml butyl acetate and placed in a 100ml three-neck flask, 1.1g (0.0069mol) bromine was dissolved in 5ml butyl acetate, and slowly Add dropwise to the reaction solution, TLC monitors the reaction end point, add water and stir for 15 minutes, separate layers, extract with ethyl acetate, wash with water until neutral, dry, and dissolve, the reddish-brown oily substance, crystals precipitate out after adding petroleum ether, filter, and dry to obtain 1.1 g of light yellow crystals (compound III), yield 78.6%
Embodiment 2
[0024] Embodiment 2: the synthesis of compound IV
[0025] Add 0.1g (0.37mmol) 2-bromo-1-(2,5-dimethoxyphenyl) acetone (compound III), 2ml ethanol, 0.16g (0.73mmol) bis-tert-butylcarbonylamine to a 50ml flask, 1ml of 8% NaOH solution was heated to reflux, and the end point of the reaction was monitored by TLC. Add water and stir for 15 minutes, extract with ethyl acetate, wash with water, dry, and dissolve to obtain 0.13 g of yellow solid (Compound IV), yield 86.67%
Embodiment 3
[0026] Embodiment 3: the synthesis of compound V
[0027] Add 0.17g (0.4mmol) 2-bis-tert-butylcarbonylamine-1-(2,5-dimethoxybenzene) acetone (compound IV) to a 50ml flask, add 0.06g (1.7mmol) in batches to 20ml ethanol ) sodium borohydride, stirred at room temperature, TLC monitored the reaction end point, the mixture was poured into water and stirred for 15 minutes, extracted with ethyl acetate, washed with water, dried and precipitated to obtain 0.14 g of light yellow solid (Compound V), yield 82.35%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com