Preparation method of bacterial cellulose capable of removing endotoxin
A technology of bacterial cellulose and bacterial cellulose membrane, which is applied in the field of bacterial cellulose preparation, can solve the problems of high price, limited sample size, and difficult removal of endotoxin, etc., and achieves the effect of simple preparation process and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The commercially available bacterial cellulose membrane prepared by Acetobacter xylinum was washed with purified water several times to control the pH value of the bacterial cellulose to 7-7.2 to obtain a pretreated bacterial cellulose membrane, which was refrigerated for future use.
[0028] Prepare 40%wt PEG200 aqueous solution with purified water.
[0029] Soak the bacterial cellulose membrane in PEG aqueous solution, heat it in a water bath to 80°C, and keep it warm for 0.5h.
[0030] After cooling, the PEG / bacterial cellulose composite membrane was taken out and washed 3 times with purified water.
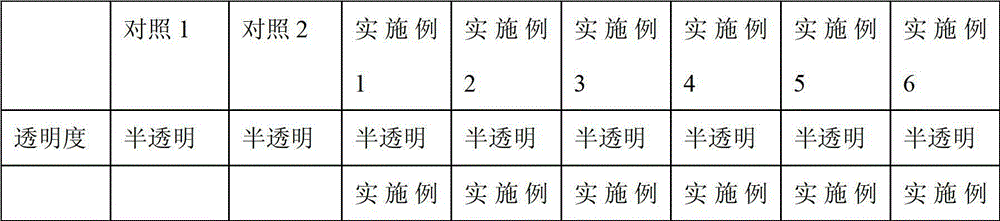
[0031] For endotoxin detection, the bacterial endotoxin limit is not more than 20EU per piece, and the sensitivity of the LAL reagent is 1EU / ml.
Embodiment 2
[0033] The commercially available bacterial cellulose membrane prepared by Acetobacter xylinum was washed with purified water several times to control the pH value of the bacterial cellulose to 7-7.2 to obtain a pretreated bacterial cellulose membrane, which was refrigerated for future use.
[0034] Prepare 50%wt PEG400 aqueous solution with purified water.
[0035] Soak the bacterial cellulose membrane in PEG aqueous solution, heat it in a water bath to 90°C, and keep it warm for 1h.
[0036] After cooling, the PEG / bacterial cellulose composite membrane was taken out and washed 3 times with purified water.
[0037] The obtained PEG / bacterial cellulose composite membrane was sterilized by high temperature and moist heat.
[0038] For endotoxin detection, the bacterial endotoxin limit is not more than 20EU per piece, and the sensitivity of the LAL reagent is 1EU / ml.
Embodiment 3
[0040] The commercially available bacterial cellulose membrane prepared by Acetobacter xylinum was washed with purified water several times to control the pH value of the bacterial cellulose to 7-7.2 to obtain a pretreated bacterial cellulose membrane, which was refrigerated for future use.
[0041] Prepare 75%wt PEG800 aqueous solution with purified water.
[0042] Soak the bacterial cellulose membrane in PEG aqueous solution, heat it in a water bath to 100°C, and keep it warm for 1.5h.
[0043] After cooling, the PEG / bacterial cellulose composite membrane was taken out and washed 3 times with purified water.
[0044] The obtained PEG / bacterial cellulose composite membrane was sterilized by irradiation, and the dose of irradiation sterilization was 5KGy.
[0045] For endotoxin detection, the limit of bacterial endotoxin is not more than 2.15EU per piece, and the sensitivity of LAL is 0.25EU / ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com