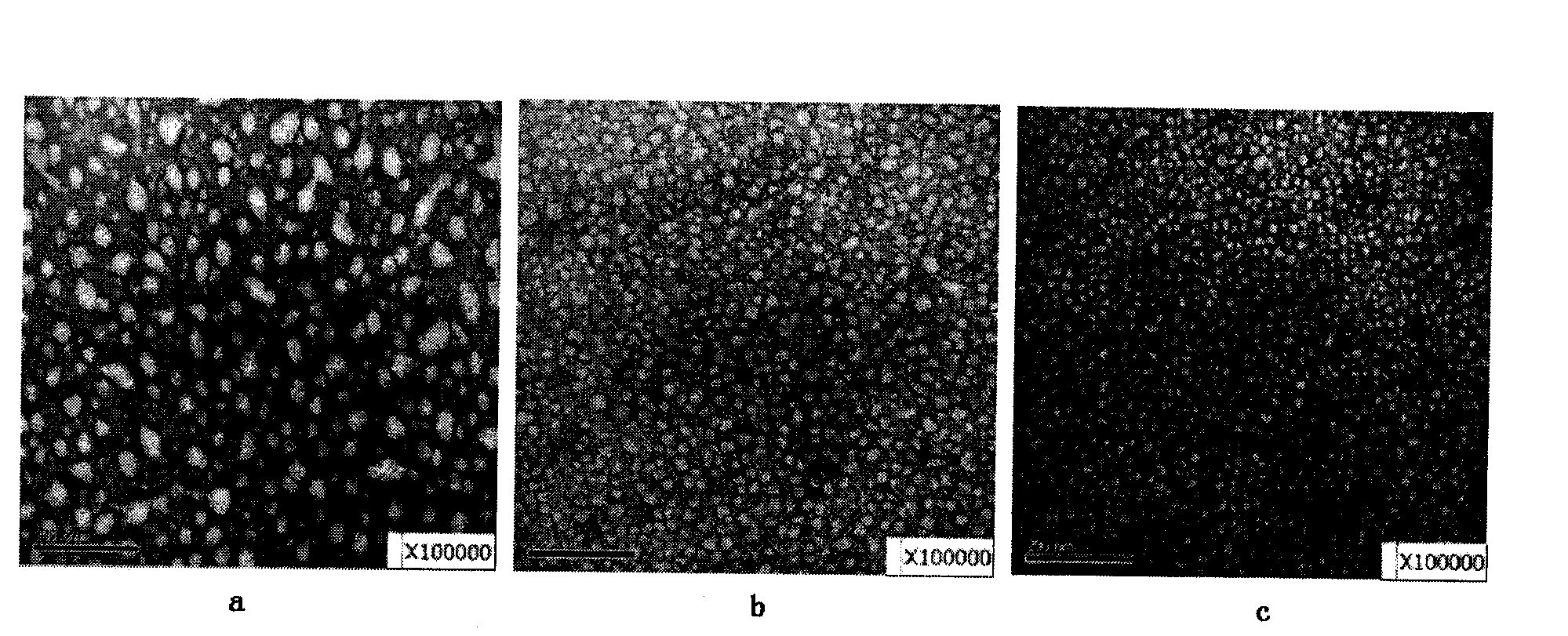
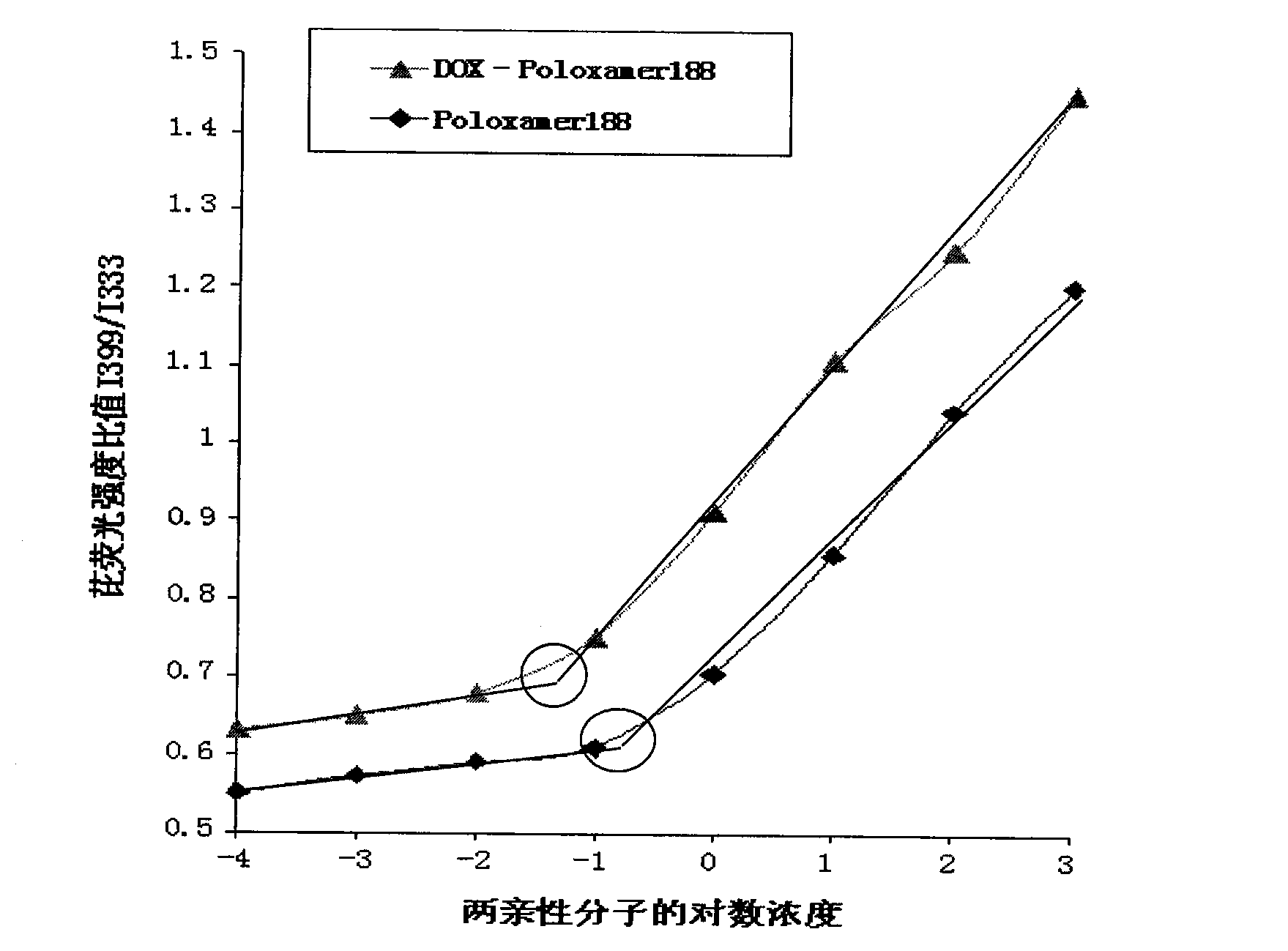
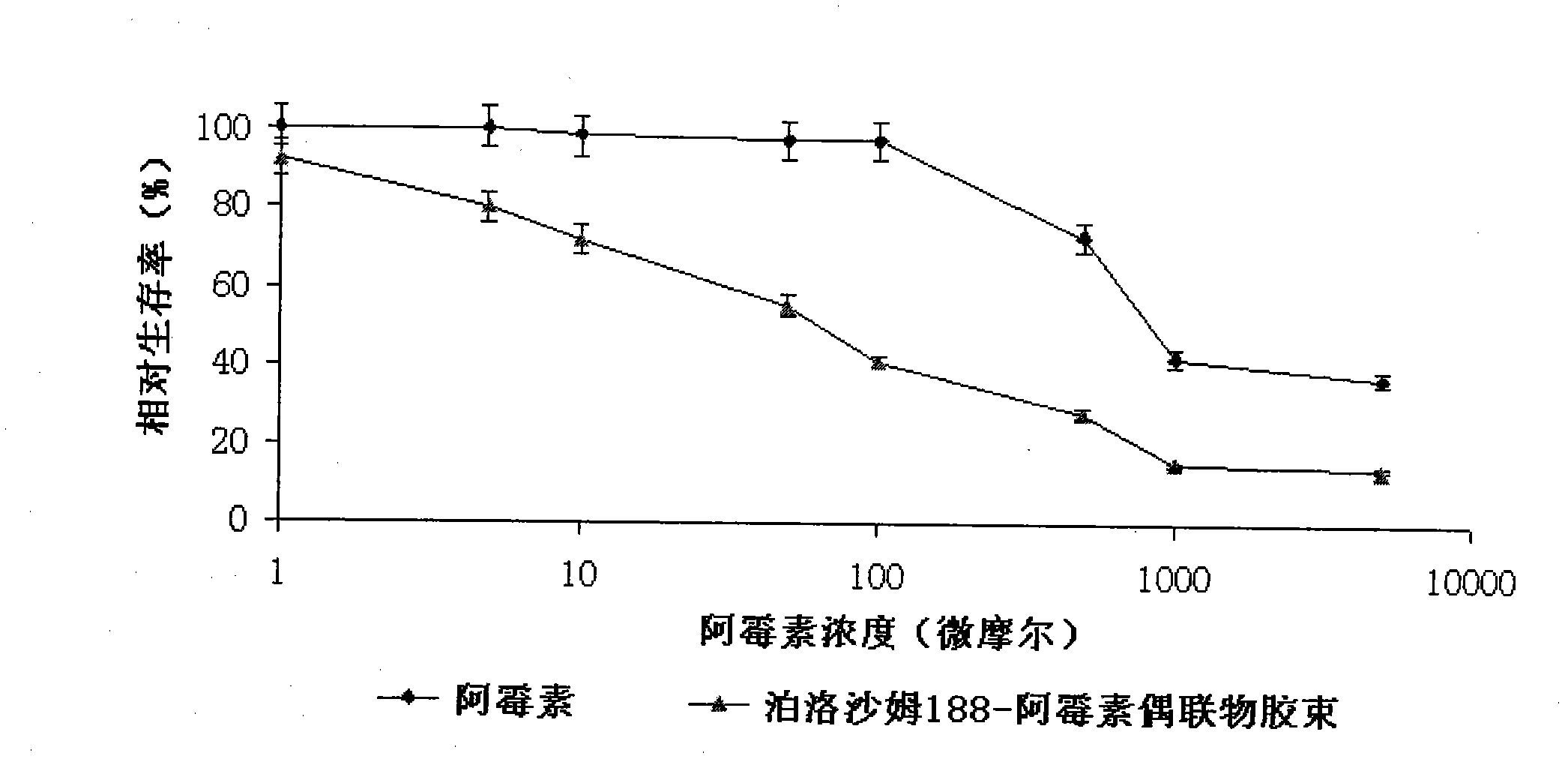
Poloxamer-adriamycin conjugate with anti-tumor effect and preparation method thereof
A technology of anti-tumor effect and doxorubicin, which is applied in the direction of anti-tumor drugs, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc. It can solve the problems of accumulation and inability to degrade polyethylene glycol, etc. Achieve the effect of high encapsulation rate, good stability and enhanced amphiphilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Synthesis of Poloxamer 188-Adriamycin Conjugate
[0030] Synthesis of poloxamer 188-doxorubicin conjugate: take 10mmol poloxamer 188, 25mmol succinic anhydride, 20mmol DMAP, 20mmol ethylenediamine, and use 30ml dioxane as an organic solvent, and stir at room temperature for 24h. After the reaction, the reaction solution was transferred to a dialysis bag with a molecular weight cut-off of 7kD, dialyzed for 48 hours using dioxane as the dialysis medium, and freeze-dried to obtain a poloxamer polymer with a carboxyl group at the end; take 10 mmol of carboxyl group at the end Poloxamer polymer, 20mmol doxorubicin, 15mmol EDC, 15mmol NHS, dissolved in 30ml N, N-dimethylformamide (DMF), protected from light, under nitrogen protection, stirred at room temperature for 24h; after the reaction Transfer the reaction solution to a dialysis bag, use 1000ml DMF as the dialysis medium, and dialyze for 24 hours, and change the dialysis medium every 6 hours during the dialysis...
Embodiment 2
[0031] Example 2 Synthesis of Poloxamer 188-Adriamycin Conjugate
[0032]Synthesis of poloxamer 188-doxorubicin conjugate: take 10mmol poloxamer 188, 25mmol succinic anhydride, 20mmol DMAP, 20mmol ethylenediamine, and use 30ml dioxane as an organic solvent, and stir at room temperature for 24h. After the reaction, the reaction solution was transferred to a dialysis bag with a molecular weight cut-off of 7kD, dialyzed for 48 hours using dioxane as the dialysis medium, and freeze-dried to obtain a poloxamer polymer with a carboxyl group at the end; take 10 mmol of carboxyl group at the end Poloxamer polymer, 30mmol doxorubicin, 15mmol EDC, 15mmol NHS, dissolved in 30ml N,N-dimethylformamide (DMF), protected from light, under nitrogen protection, stirred at room temperature for 24h; after the reaction Transfer the reaction solution to a dialysis bag, use 1000ml DMF as the dialysis medium, and dialyze for 24 hours, and change the dialysis medium every 6 hours during the dialysis p...
Embodiment 3
[0033] Example 3 Synthesis of Poloxamer 188-Adriamycin Conjugate
[0034] Synthesis of poloxamer 188-doxorubicin conjugate: take 10mmol poloxamer 188, 25mmol succinic anhydride, 20mmol DMAP, 20mmol ethylenediamine, and use 30ml dioxane as an organic solvent, and stir at room temperature for 24h. After the reaction, the reaction solution was transferred to a dialysis bag with a molecular weight cut-off of 7kD, dialyzed for 48 hours using dioxane as the dialysis medium, and freeze-dried to obtain a poloxamer polymer with a carboxyl group at the end; take 10 mmol of carboxyl group at the end Poloxamer polymer, 5mmol doxorubicin, 15mmol EDC, 15mmol NHS, dissolved in 30ml N,N-dimethylformamide (DMF), protected from light, under nitrogen protection, stirred at room temperature for 24h; after the reaction Transfer the reaction solution to a dialysis bag, use 1000ml DMF as the dialysis medium, and dialyze for 24 hours, and change the dialysis medium every 6 hours during the dialysis p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com