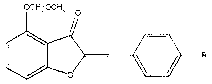
Preparation method of 4-hydroxy aurone compound
A compound, the technology of dihydroxyacetophenone, which is applied in the field of synthesis of hydroxyuranone compounds, can solve the problems such as the method for preparing 4-hydroxyuranone compounds is not clearly disclosed, the reaction time is long, the reaction steps are cumbersome and the like, and the product yield is achieved. The effect of high, short reaction time and low raw material price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
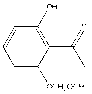
[0040] (1) Preparation of 2'-hydroxy-6'-methoxymethoxyacetophenone
[0041] With 4.50g (29.6mmol) 2', 6'-dihydroxyacetophenone, 8.46g (61.3mmol) anhydrous K 2 CO 3 , 73.2mL of acetone was put into a 100mL two-necked bottle, and stirred vigorously for 1h in an oil bath at 50°C. Then, slowly dropwise added 2.4mL (30 mmol) chloromethyl methyl ether into the two-necked flask, and continued the reaction for 5h after the dropwise addition. The reaction solution was filtered, and the residue was rinsed with a small amount of acetone; the filtrate and washings were collected and evaporated to dryness to remove acetone. Using a mixture of petroleum ether and ethyl acetate as the eluent, pass through a silica gel chromatography column to obtain 4.80 g of a colorless oily liquid, namely 2'-hydroxy-6'-methoxymethoxyacetophenone, with a yield of 82.7% . Among them, V (petroleum ether) / V (ethyl acetate)=10:1.
[0042] (2) Preparation of 2'-hydroxy-6'-methoxymethoxy-4''-N, N-dimethylami...
Embodiment 2
[0053] (1) Using the method of step (1) in Example 1 to prepare 2'-hydroxy-6'-methoxymethoxyacetophenone;
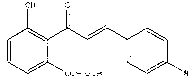
[0054] (2) Add 0.49g (3.3mmol) 4-(N,N-dimethylamino)benzaldehyde, 0.65g (3.3mmol) 2'-hydroxy-6'-methoxymethoxyacetophenone into 30mL Then add 20 mL of 10% sodium hydroxide ethanol solution dropwise, and stir at room temperature for 18 h. Then, the product in the flask was poured into ice water, stirred, and allowed to stand. After the layers were separated, the upper layer was poured off (removing ethanol); then 90 mL of dichloromethane was added for extraction. Purify the extract with a silica gel chromatography column, the eluent is a mixture of dichloromethane and petroleum ether; the collected components are evaporated to dryness to obtain a red solid (2'-hydroxy-6'-methoxymethoxy Base-4''-N, N-dimethylamino-chalcone) 0.48g, yield 44.1%. Wherein, V (methylene chloride) / V (petroleum ether)=1:1.
[0055] (3) Using the method of step (3) in Example 1 to prepare 4'-N,...
Embodiment 3
[0058] (1) Preparation of 2'-hydroxy-6'-methoxymethoxyacetophenone
[0059] With 4.50g (29.6mmol) 2', 6'-dihydroxyacetophenone, 8.46g (61.3mmol) anhydrous K 2 CO 3 , 73.2mL of anhydrous acetone was put into a 100mL two-necked bottle, and stirred vigorously for 1h under the condition of an oil bath at 55°C. Then, 6.0 mL (78 mmol) of chloromethyl methyl ether was slowly added dropwise into the two-necked flask, and the reaction was continued for 6 h after the dropwise addition. The reaction solution was filtered, and the residue was rinsed with a small amount of acetone; the filtrate and washings were collected and evaporated to dryness to remove acetone. Using a mixture of petroleum ether and ethyl acetate as the eluent, pass through a silica gel chromatography column to obtain 5.62 g of a colorless oily liquid, which is 2'-hydroxy-6'-methoxymethoxyacetophenone, with a yield of 96.8% . Among them, V (petroleum ether) / V (ethyl acetate)=10:1.
[0060] (2) Preparation of 2'-h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com