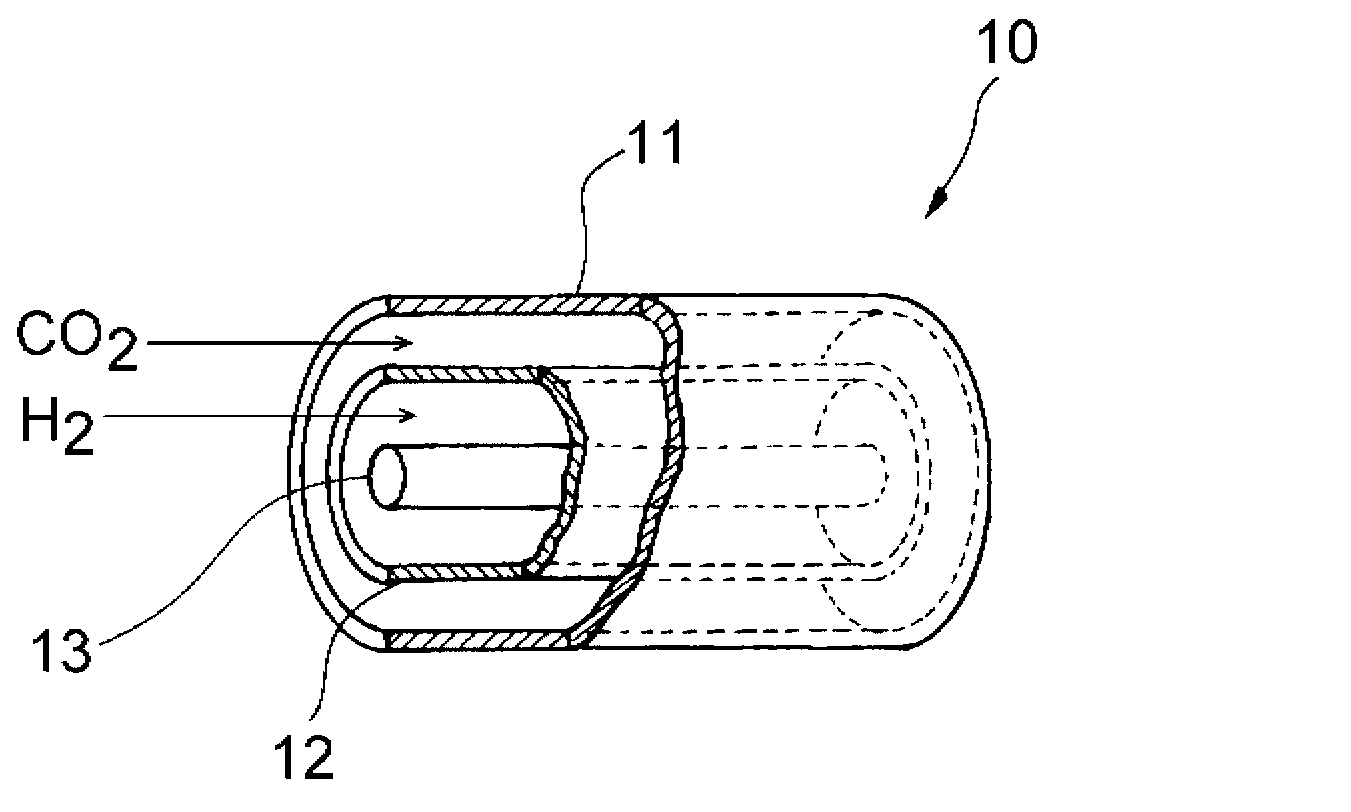
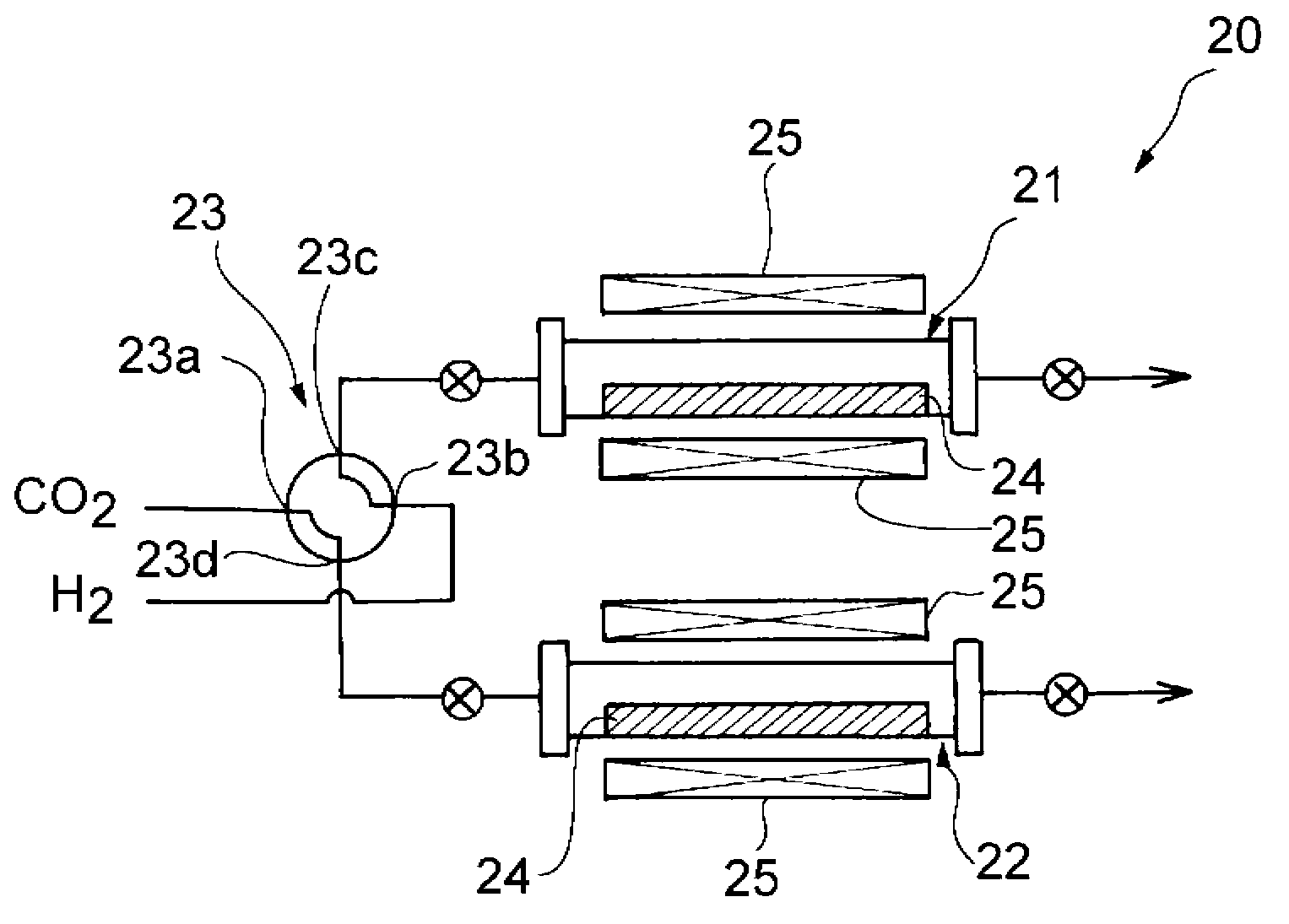
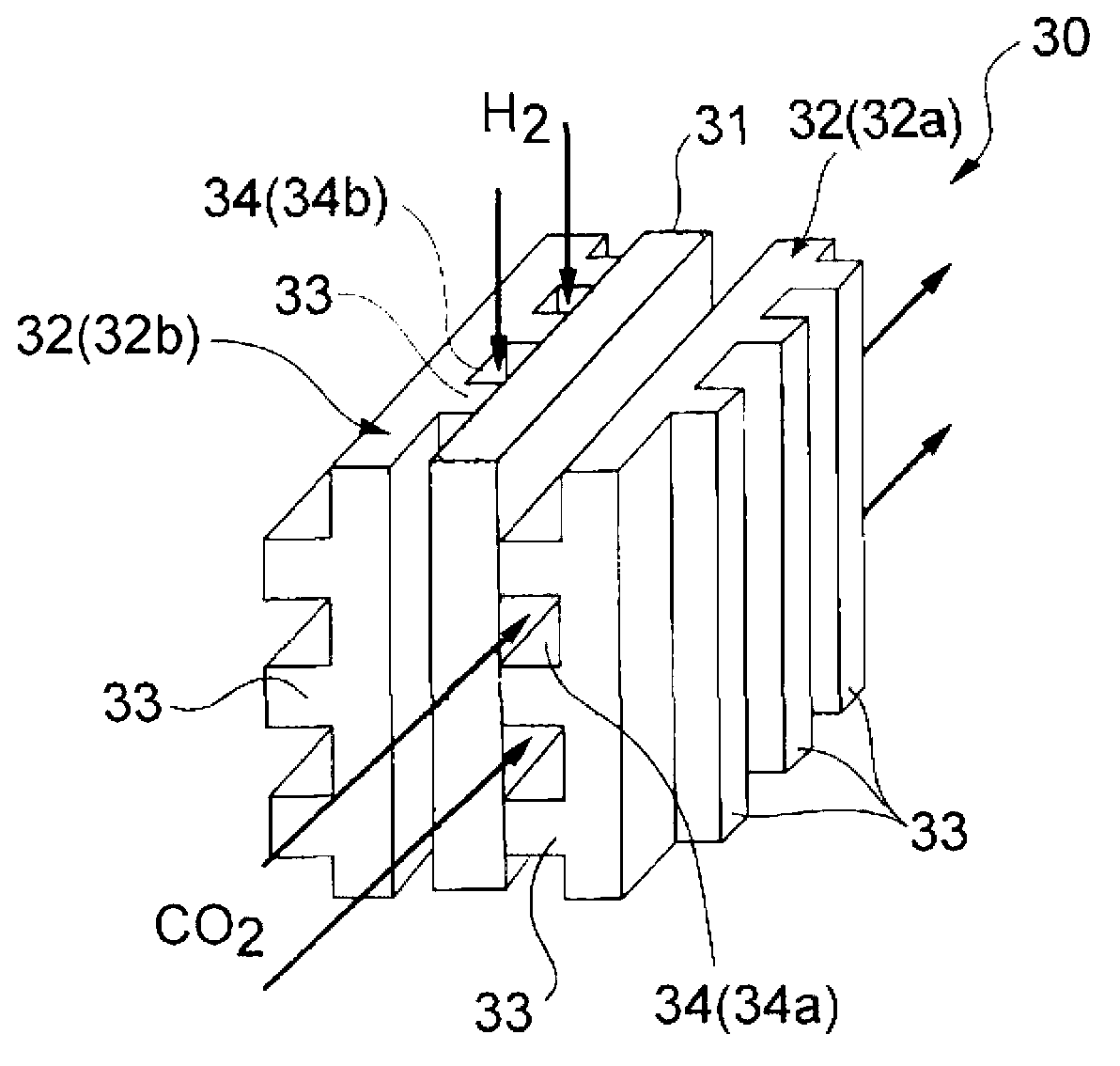
Method for producing carbon monoxide and production apparatus
A manufacturing method and carbon monoxide technology, applied in the direction of carbon monoxide, energy input, etc., to achieve the effect of reducing temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] (1) Production of lanthanum-containing cerium oxide with reversible oxygen deficiency
[0078] (A) Synthesis of cerium oxide containing lanthanum
[0079] Powder of composite oxide of cerium oxide-lanthanum oxide is used. This composite oxide is an oxide prepared so that the ratio of the number of moles of lanthanum to the total amount of the number of moles of cerium and lanthanum becomes 0.2. 50 g of the composite oxide was allowed to stand still in a heating furnace, and heated while circulating air for firing. The heating was started from room temperature and heated at a temperature increase rate of 5°C / min. After reaching 1000°C, the temperature was maintained for 2 hours. The air flow rate is set to 0.5L / min. Then, it was allowed to cool naturally to obtain lanthanum-containing ceria without reversible oxygen defects. In the measurement using XRD, no La 2 O 3 Diffraction peaks, only observed from CeO 2 Diffraction peaks. From this result, it was confirmed that lan...
Embodiment 2
[0104] In Example 2, the praseodymium-containing cerium oxide that does not have reversible oxygen defects was not subjected to hydrogen reduction, and the conversion evaluation from carbon dioxide gas to carbon monoxide gas was directly performed. In addition, for the praseodymium-containing cerium oxide, the reversible oxygen defect generation process using reducing gas is not performed, and it directly reacts with carbon dioxide gas to measure T CO2 . The results are shown in Table 1 below.
[0105] Table 1
[0106]
[0107] As indicated by the results shown in Table 1, it was found that carbon dioxide can be converted into carbon monoxide by using the conversion agent obtained in each example. In particular, as shown by the comparison between Examples 1-7 and Reference Example 1 at a temperature of 400°C, it was found that by adding rare earth elements such as lanthanum (excluding cerium) to cerium oxide, carbon monoxide is generated from carbon dioxide even at a lower tempe...
Embodiment 8 and 9
[0110] As the composite oxide of cerium oxide-lanthanum oxide, an oxide prepared so that the ratio of the moles of lanthanum to the total moles of cerium and lanthanum reaches the value shown in Table 2 was used. 1 In the same manner, the lanthanum-containing ceria containing no reversible oxygen deficiency of Examples 8 and 9 was obtained. In the measurement by XRD, the lanthanum-containing cerium oxides of Examples 8 and 9 without reversible oxygen defects were not observed to be derived from La 2 O 3 Diffraction peaks, only observed from CeO 2 Diffraction peaks. From this result, it was confirmed that lanthanum was dissolved in cerium oxide. In the same manner as in Example 1, from the lanthanum-containing cerium oxide having no reversible oxygen deficiency, the lanthanum-containing cerium oxide having reversible oxygen deficiency of Examples 8 and 9 was obtained. For the obtained lanthanum-containing cerium oxide without reversible oxygen defects, the same T as in Example ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com