Compound preparation of valsartan amlodipine tablet (I) and preparation method thereof
A technology for valsartan amlodipine tablet and compound preparation, which is applied in the field of biomedicine, can solve the problems of uneven drug content, easy introduction of impurities, etc. reasonable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
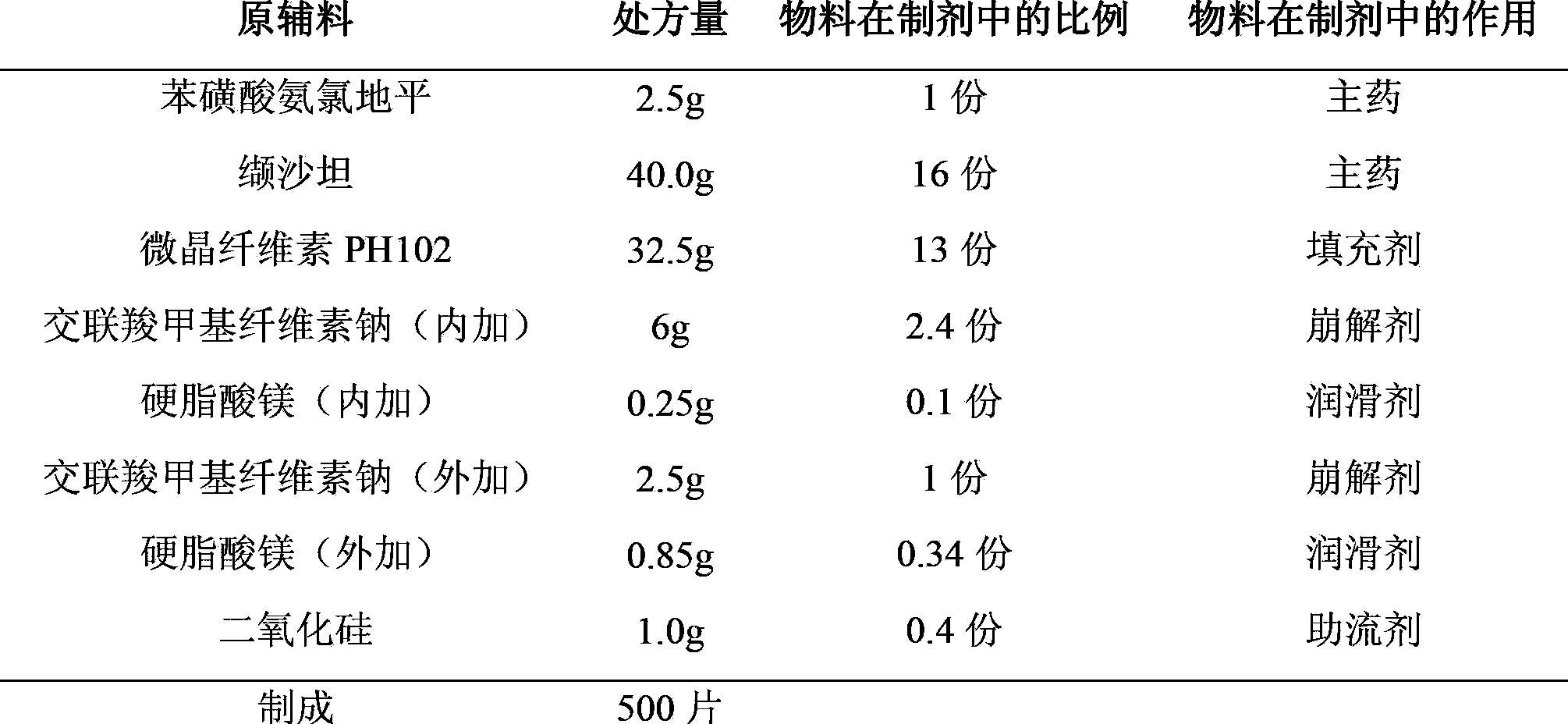
[0031] Plain Tablet Prescription:
[0032]
[0033] Formulation of coating solution for film coating layer:
[0034]
[0035]
[0036] Preparation:
[0037] (1) Pass amlodipine besylate and valsartan through a 100-mesh sieve and silicon dioxide through a 80-mesh sieve respectively, and set aside.
[0038] (2) Weigh the prescribed amount of amlodipine besylate, the prescribed amount of croscarmellose sodium (internal addition), and microcrystalline cellulose PH102, and mix them uniformly by incremental method, and pass through a 40-mesh sieve; mix the powder and the prescribed amount Add valsartan and magnesium stearate (internal addition) into the mixer and mix for about 10 minutes.
[0039] (3) Granulate the mixed powder with a dry granulator, set the speed of the roller to about 5 rpm, the speed of feeding to 28 rpm, and the pressure of the roller to 1.0T; sieve the particles between 24 mesh and 60 mesh In the next step, the particles larger than 24 mesh are gran...
Embodiment 2
[0043] Plain Tablet Prescription:
[0044]
[0045] Formulation of coating solution for film coating layer:
[0046]
[0047] Preparation:
[0048] (1) Pass amlodipine besylate and valsartan through a 100-mesh sieve respectively, and set aside.
[0049] (2) Weigh the prescribed amount of amlodipine besylate, the prescribed amount of croscarmellose sodium (internal addition), and microcrystalline cellulose PH102, mix evenly by adding method, and pass through a 40-mesh sieve; mix the mixed powder and the prescribed amount Add valsartan and magnesium stearate (internal addition) into the wet mixer, add 10% aqueous solution of sodium carboxymethylcellulose, make soft material, granulate with 24 mesh, dry, and granulate with 22 mesh.
[0050] (3) Measure the content of intermediates. After passing the test, add magnesium stearate (external addition) and croscarmellose sodium (external addition), mix for 15 minutes, and press into tablets.
[0051] (4) Slowly add the film c...
Embodiment 3
[0053] Plain Tablet Prescription:
[0054]
[0055] Formulation of coating solution for film coating layer:
[0056]
[0057] Preparation:
[0058] (1) Pass amlodipine besylate and valsartan through a 100-mesh sieve and silicon dioxide through a 80-mesh sieve respectively, and set aside.
[0059] (2) Weigh the prescribed amount of amlodipine besylate, the prescribed amount of croscarmellose sodium (internal addition), and microcrystalline cellulose PH102, and mix them uniformly by incremental method, and pass through a 40-mesh sieve; mix the powder and the prescribed amount Add valsartan and magnesium stearate (internal addition) into the mixer and mix for about 10 minutes.
[0060] (3) Granulate the mixed powder with a dry granulator, set the speed of the roller to about 4 rpm, the speed of feeding to 24 rpm, and the pressure of the roller to 0.8T; sieve the particles between 24 mesh and 60 mesh, Granules larger than 24 mesh are granulated with a 24-mesh screen of a sw...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com