Caprolactam production method
A technology for caprolactam and cyclohexanone, applied in the field of caprolactam production, can solve the problems of large consumption of oleum, low reaction product yield, complicated refining system, etc., saving construction investment and production cost, high product yield, The stable effect of the preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0013] The present invention will be further described below in conjunction with the drawings.
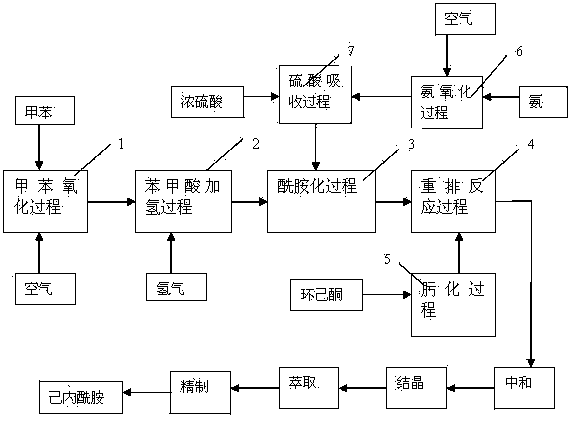
[0014] The invention produces caprolactam such as figure 1 As shown, it mainly includes toluene oxidation process 1, benzoic acid hydrogenation process 2, amidation process 3, rearrangement reaction process 4, oximation process 5, ammoxidation process 6, and sulfuric acid absorption process 7. Toluene oxidation process 1 uses toluene as a raw material and oxidizes to benzoic acid with air under the action of a cobalt acetate catalyst. The reaction pressure is 1000KPa, the reaction temperature is 170±5°C, the single-pass conversion rate of the reaction is 15%, and the benzoic acid selectivity is 92%. In benzoic acid hydrogenation process 2, benzoic acid is hydrogenated to cyclohexane carboxylic acid (CCA) under the action of ruthenium-carbon catalyst. The reaction pressure is 1500KPa, and the reaction temperature is 170±5℃. The conversion rate and CCA selectivity of the reaction are cl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com