Alkaline polyarylether ionomer material with microphase separation structure and preparation and application thereof
A technology of microphase separation and polyarylether, which is applied in the field of basic polyarylether ionomer and its preparation, can solve the problems of decreased alcohol resistance performance, decreased long-term chemical stability in the working environment, and decreased ion conductivity, etc., to achieve Enhanced ionic conductivity, good ion-conducting performance, and high ionic conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The present embodiment adopts following method to prepare basic polyarylether ionomer:
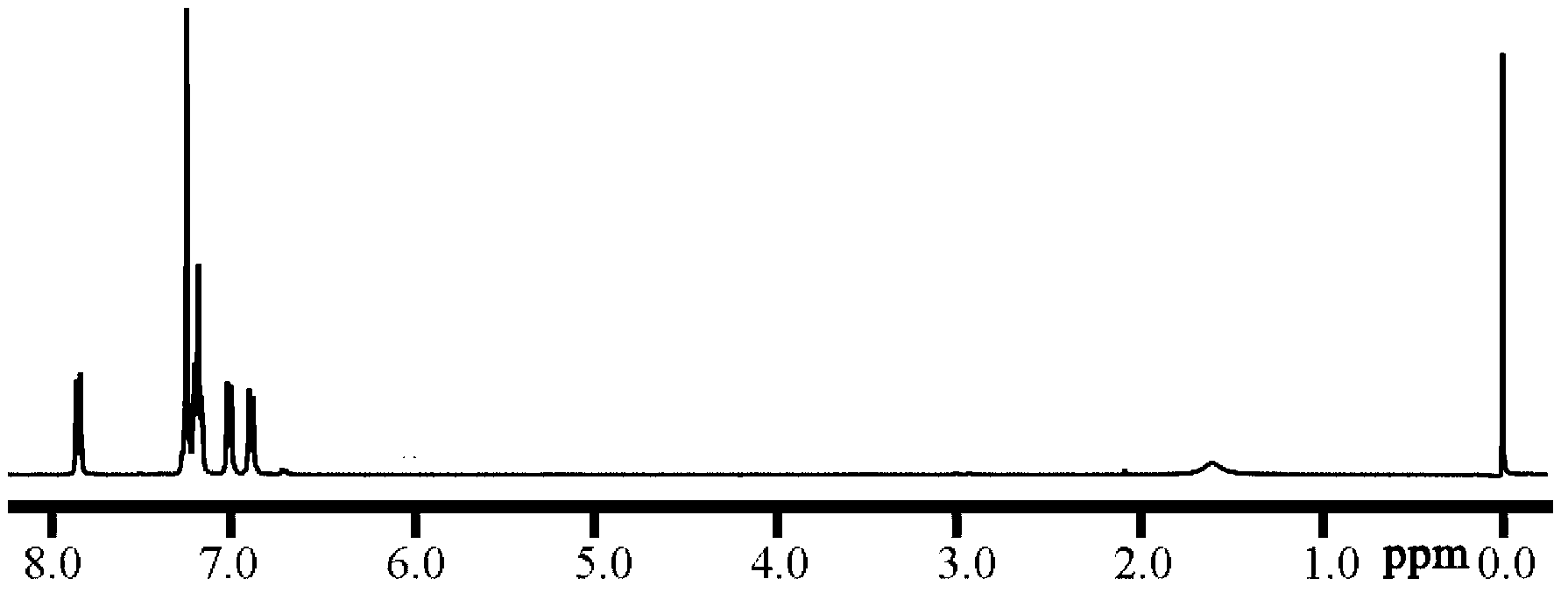
[0054] 1) Synthesis of hydrophilic segment: 7.5183g (21.333mmol) (ie m+1=16) of bis(4,4'-hydroxyphenyl) diphenylmethane, 5.085g (20mmol) (ie m=15) ) of dihalogen monomer a1, 4.4417g (32.142mmol) of anhydrous potassium carbonate, 30mL N,N-dimethylacetamide and 30mL toluene were added to a three-necked flask, and under nitrogen protection, the temperature of the oil bath was 140°C under reflux water for 3 hours, then warmed up to 180°C, after 12 hours of reaction, add 0.3759g (1.0666mmol) (ie 5mol% (m+1)) of bis(4,4'-hydroxyphenyl) diphenylmethane, in Continue to react at 180°C for 3 hours, then cool to room temperature, precipitate the reaction solution in 60mL of 1:1 (v / v) methanol / 3% concentrated hydrochloric acid solution, dry the crude product at 80°C in vacuum for 24 hours, and then use chloroform solvent Dissolve, pass through diatomaceous earth, precipitate in methanol, colle...
Embodiment 2
[0079] Except for the following features, other steps and testing methods of the preparation method of the present embodiment are the same as in Example 1:
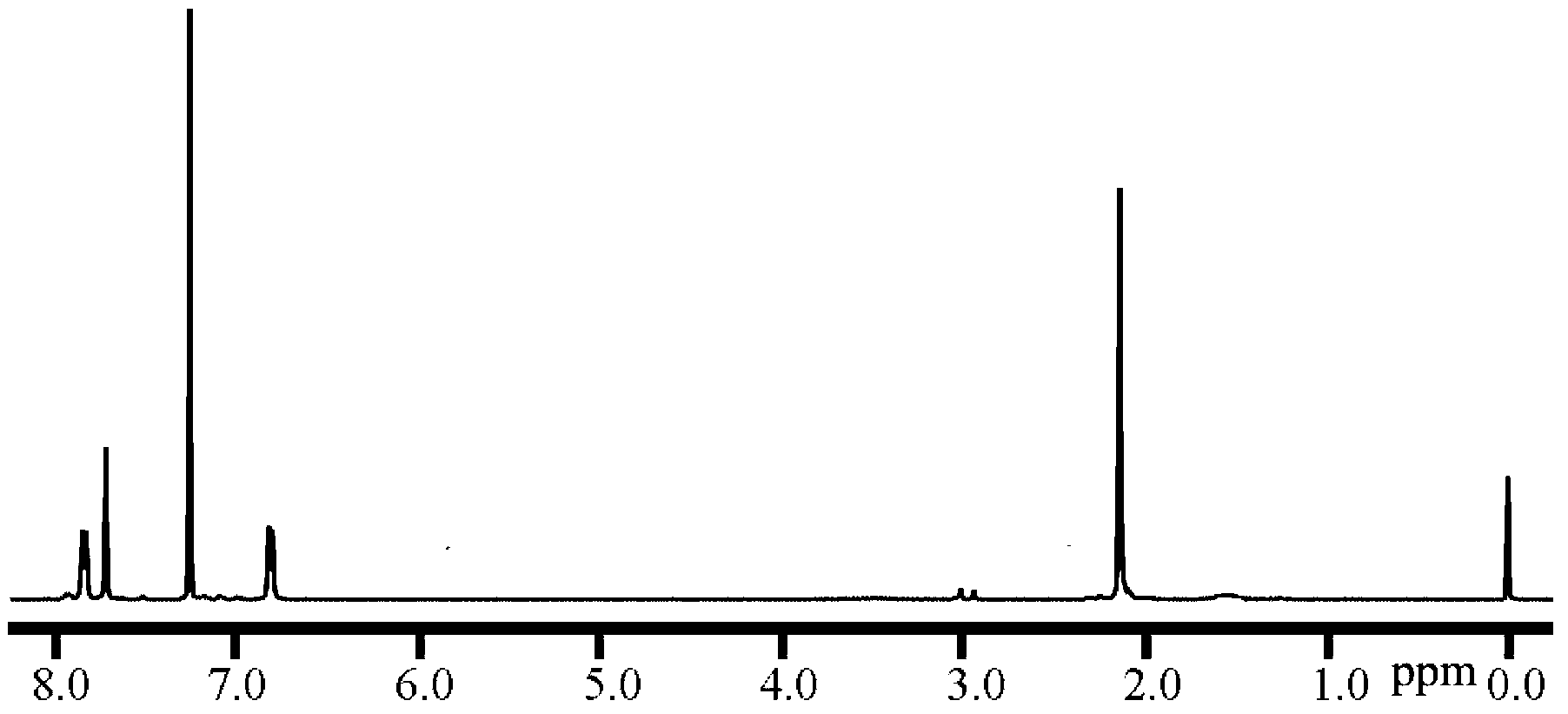
[0080] When preparing the hydrophilic segment polymer, bis(4,4'-hydroxyphenyl) diphenylmethane 7.440g (21.111mmol), dihalogen monomer b1 (where X is F) 4.364g (20mmol), m= 18, 27mL of N,N-dimethylacetamide, 30mL of toluene, under the protection of nitrogen, the temperature of the oil bath is 150°C and the water is refluxed for 6 hours, then the temperature is raised to 200°C, after 12 hours of reaction, add 5mol% ( m+1) of bis(4,4'-hydroxyphenyl)diphenylmethane, continue to react at 200°C for 3 hours, then cool to room temperature, and dissolve the reaction solution in 60mL of 1:1 (v / v) methanol / 3% concentrated hydrochloric acid solution precipitation, the crude product was dried in vacuum at 80°C for 24 hours, then dissolved in chloroform solvent, passed through diatomaceous earth, precipitated in methanol, collected th...
Embodiment 3
[0105] Except for the following features, other steps and testing methods of the preparation method of the present embodiment are the same as in Example 2:
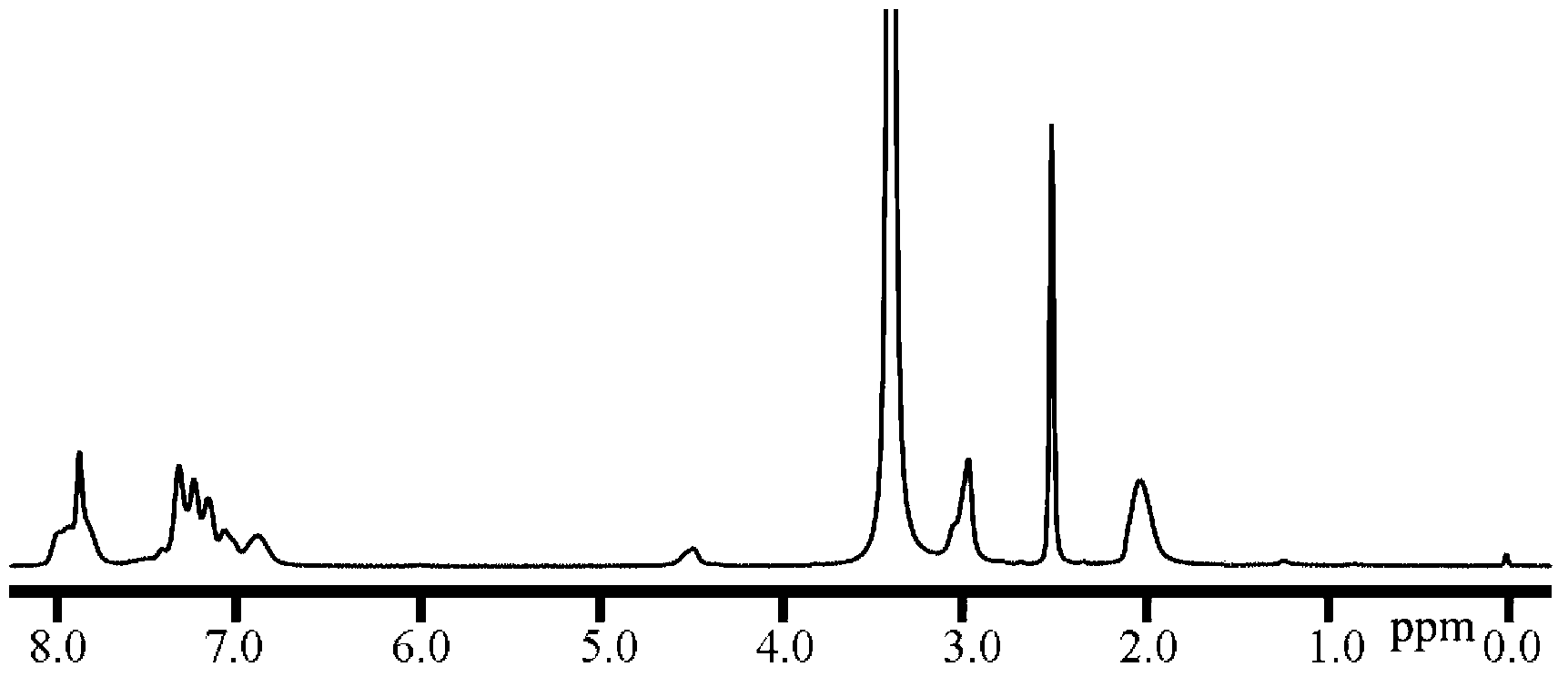
[0106] When preparing the hydrophilic segment polymer, the reactant is 7.552g (21.4285mmol) of bis(4,4'-hydroxyphenyl) diphenylmethane, 8.8288g (20mmol) of dihalogen monomer c1 (where X is Cl) , m=14, N,N-dimethylacetamide is 37mL, and toluene is 60mL.
[0107] The dihalogen monomer in this embodiment has the following structure:
[0108]
[0109] Wherein X is Cl;
[0110] The hydrophilic segment of the present embodiment has the following structure:
[0111]
[0112] has the following structure:
[0113]
[0114] When preparing hydrophobic segment polymers, the reactant dihalogen monomer c1 (where X is Cl) 8.8799g (21.4285mmol), dihydroxy aromatic monomer I5.0054g (20mmol), n=14, dimethyl sulfoxide 32mL , Toluene 60mL.
[0115] The dihydroxyaromatic monomer of the present embodiment has the following stru...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ion exchange capacity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Ionic conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com