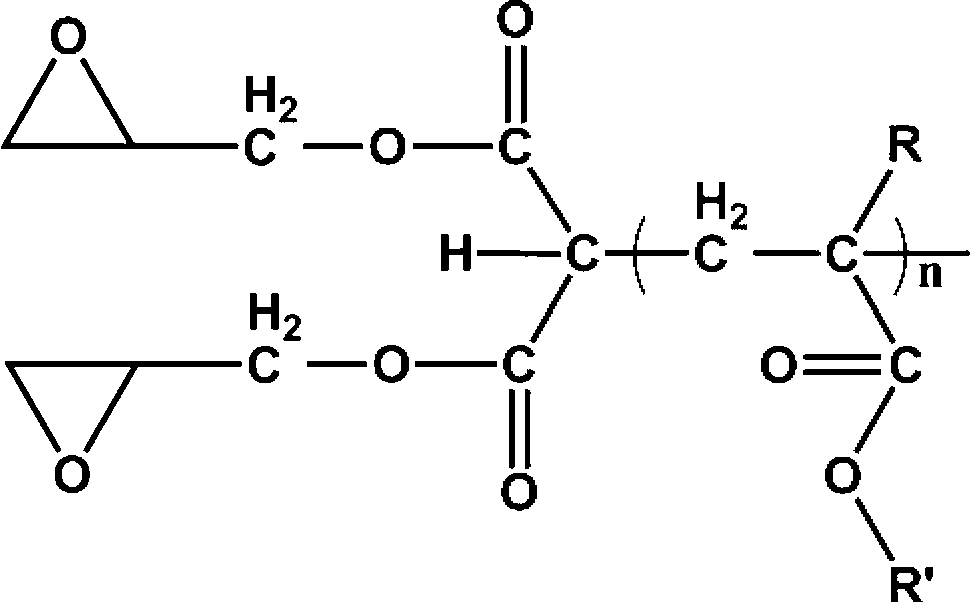
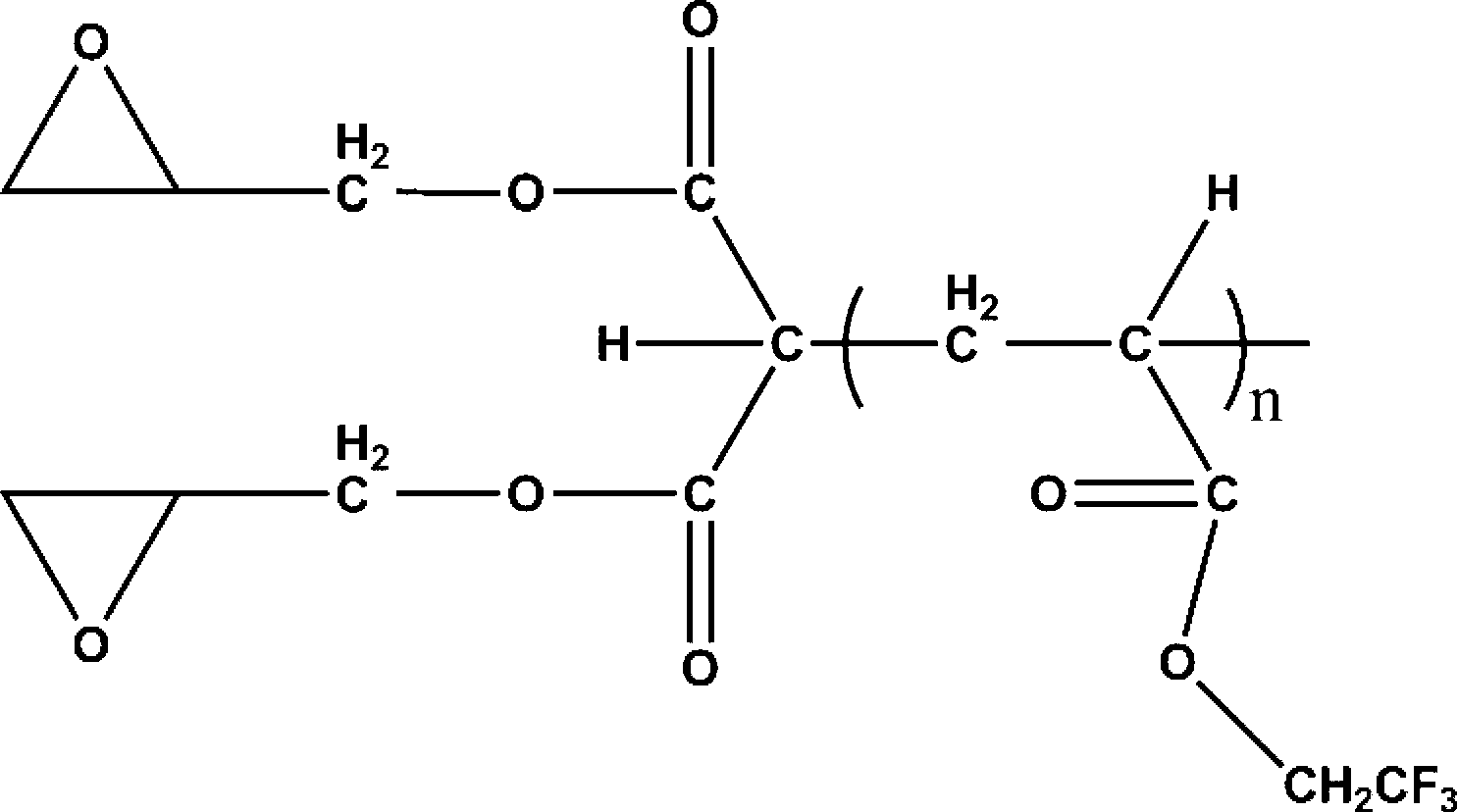
Fluorine-containing epoxy composite cation photocureable coating as well as preparation method and application thereof
A technology of light-curing coatings and cations, applied in the direction of epoxy resin coatings, coatings, etc., can solve the problems of high cost, coating hydrophobicity, anti-fouling performance, complex synthesis methods, etc., to improve compatibility, The effect of excellent hydrophobic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) Mix 0.1 parts by mass of dibutyl malonate, 50 parts by mass of toluene and 0.5 parts by mass of tetrabutylammonium hydroxide aqueous solution with a concentration of 25% by mass, react for 5 hours, and remove toluene under reduced pressure; then add 90 parts by mass part tetrahydrofuran and 5 parts by mass trifluoroethyl acrylate monomer, mixed uniformly and reacted for 0.1h, after the tetrahydrofuran was removed under reduced pressure, the mixed solution of the obtained product at 20°C (30 parts by mass trifluoroacetic acid and 60 parts by mass dioxygen Hexacyclic) hydrolyzed for 4.5h to obtain a fluorine-containing polymer containing a carboxyl-terminated group.
[0038] (2) Add 2 mass parts of carboxyl-terminated fluoropolymer prepared in step (1) into 150 mass parts of tetrahydrofuran and mix evenly, then add 4 mass parts of glycidol, 5 mass parts of dehydrating agent (N,N'- Dicyclohexylcarbodiimide, DCC) and 1.2 mass parts of catalyst (4-dimethylaminopyridine, ...
Embodiment 2
[0044] (1) Mix 2.8 parts by mass of dibutyl malonate, 150 parts by mass of toluene, and 20 parts by mass of tetrabutylammonium hydroxide aqueous solution with a concentration of 25% by mass, react for 1 hour, remove toluene under reduced pressure, and then add 100 parts by mass Parts of tetrahydrofuran and 20 parts by mass of -1H, -1H-perfluorooctyl methacrylate monomer, mixed uniformly and reacted for 3 hours, after removing tetrahydrofuran under reduced pressure, the mixed solution of the obtained product at 100°C (5 parts by mass of three Fluoroacetic acid and 57 parts by mass of dioxane) were hydrolyzed for 12 hours to obtain a fluorine-containing polymer containing terminal carboxyl groups.
[0045] (2) Add 10 parts by mass of the carboxyl-terminated fluoropolymer prepared in step (1) to 30 parts by mass of tetrahydrofuran and mix evenly, then add 1 part by mass of glycidol and 2.5 parts by mass of dehydrating agent N,N'- Dicyclohexylcarbodiimide (DCC) and 1 mass part of ...
Embodiment 3
[0051] (1) Mix 0.6 parts by mass of dibutyl malonate, 80 parts by mass of toluene and 10 parts by mass of tetrabutylammonium hydroxide aqueous solution with a concentration of 25% by mass, react for 10 hours, remove toluene under reduced pressure, and then add 80 parts by mass Tetrahydrofuran and 10 mass parts of trifluoroethyl methacrylate monomers were mixed uniformly and reacted for 2 hours. After the tetrahydrofuran was removed under reduced pressure, the mixed solution of the obtained product at 70°C (10 mass parts of trifluoroacetic acid and 30 mass parts of dioxygen Hexacyclic) hydrolyzed for 1 h to obtain a fluorine-containing polymer containing a carboxyl-terminated group.
[0052] (2) Add 2 mass parts of carboxyl-terminated fluoropolymer prepared in step (1) into 80 mass parts of tetrahydrofuran and mix evenly, then add 7 mass parts of glycidol, 1 mass part of dehydrating agent N,N'-di Cyclohexylcarbodiimide (DCC) and 0.05 parts by mass of catalyst 4-dimethylaminopyr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com