Method for preparing hydrogen in process of producing manganous-manganic oxide through electrolytic manganese metal
A technology for electrolysis of manganese metal and manganese tetroxide, applied in the production of manganese oxide/hydroxide, hydrogen, etc., can solve the problems of direct hydrogen emission and high hydrogen concentration, and achieve energy saving, low economic cost and good economic benefits Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
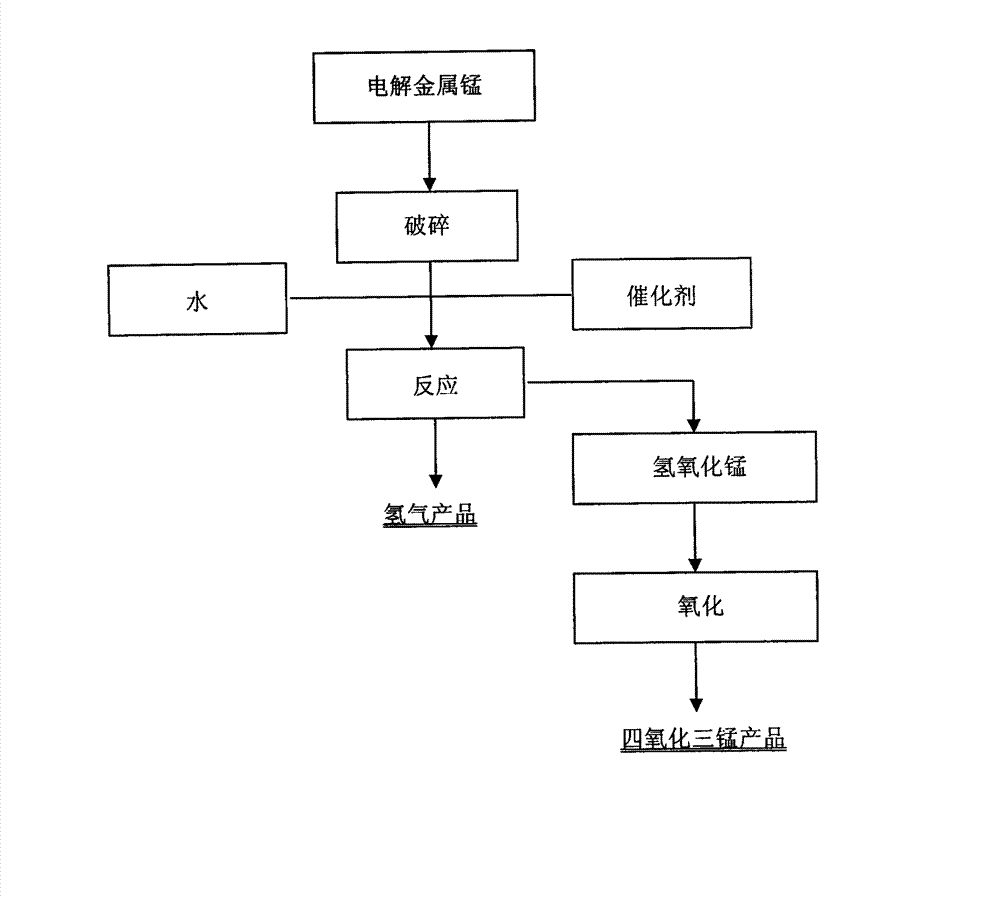
[0019] (1) Weigh 1000 g of electrolytic manganese metal, crush it in a ball mill for 1 hour to make electrolytic manganese metal powder 1, and detect that its particle size is 100% less than 100 μm with an analytical sieve;
[0020] (2) Add 3L of distilled water to electrolytic manganese metal powder 1 to prepare slurry 2, and put slurry 2 into a hydrogen generator;
[0021] (3) Weigh 10g of ammonium chloride, add it to the slurry 2, control the system temperature at 65°C, stir the reaction for 1 hour, discharge the gas released in the first 3 minutes of reaction, and use a collection bag to collect the gas 3 released in the subsequent reaction, After slurry 2 is hydrolyzed, manganese hydroxide is generated;
[0022] (4) Under the condition of temperature 60 DEG C, manganese hydroxide is passed into compressed air to carry out oxidation reaction, and after the reaction time is 6 hours, trimanganese tetraoxide 4 is obtained;
[0023] (5) Trimanganese tetraoxide 4 is washed and...
Embodiment 2
[0026] (1) Weigh 2000g of electrolytic manganese metal, crush it in a ball mill for 1.5 hours to make electrolytic manganese metal powder 1, and detect that its particle size is 100% less than 75 μm with an analytical sieve;
[0027] (2) Add 5L of distilled water to the electrolytic manganese metal powder 1 to prepare a slurry 2, and put the slurry 2 into a hydrogen generator;
[0028] (3) Weigh 25g of ammonium sulfate, add it to the slurry 2, control the temperature of the system at 80°C, stir the reaction for 1 hour, discharge the gas released in the first 3 minutes of reaction, and use a collection bag to collect the gas 3 released in the subsequent reaction, and the slurry After material 2 is hydrolyzed, manganese hydroxide is generated;
[0029] (4) Under the condition of temperature 60 DEG C, manganese hydroxide is passed into compressed air to carry out oxidation reaction, and after the reaction time is 6 hours, trimanganese tetraoxide 4 is obtained;
[0030] (5) Trima...
Embodiment 3
[0033] (1) Weigh 1500g of electrolytic manganese metal, add 4L of distilled water, and adjust it into slurry 1;
[0034] (2) Put slurry 1 into a ball mill and grind for 1 hour to make slurry 2, and put slurry 2 into a hydrogen generator;
[0035] (3) Weigh 20g of manganese chloride, add slurry 2, control the temperature of the system at 85°C, stir the reaction for 1 hour, discharge the gas released in the first 3 minutes of reaction, use a collection bag to collect the gas 3 released in the subsequent reaction, slurry After material 2 is hydrolyzed, manganese hydroxide is generated;
[0036] (4) Under the condition of temperature 60 DEG C, manganese hydroxide is passed into compressed air to carry out oxidation reaction, and after the reaction time is 6 hours, trimanganese tetraoxide 4 is obtained;
[0037] (5) Trimanganese tetraoxide 4 is washed and dried to obtain trimanganese tetraoxide product 5.
[0038] Finally, the hydrogen content of gas 3 was monitored by a portable...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com