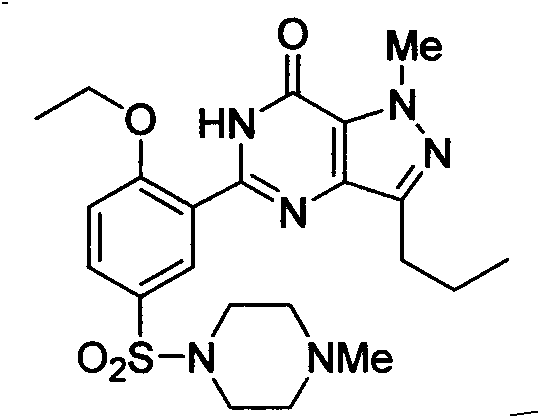
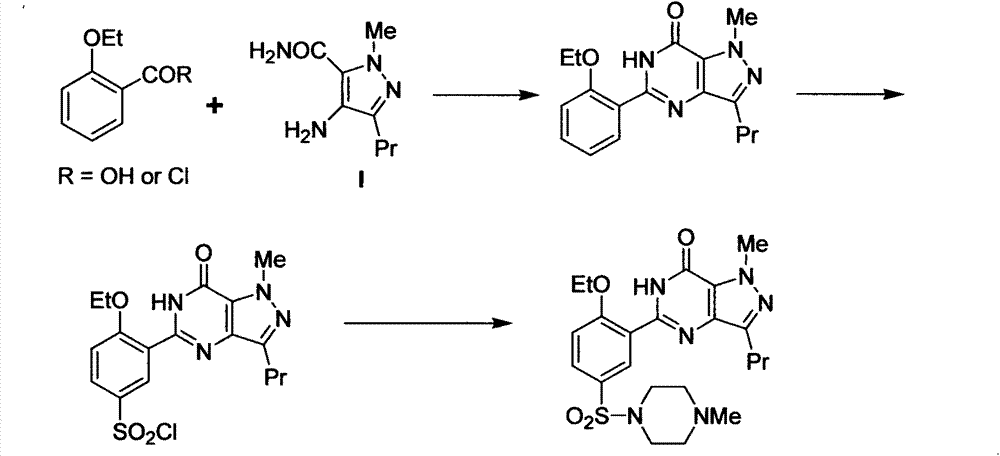
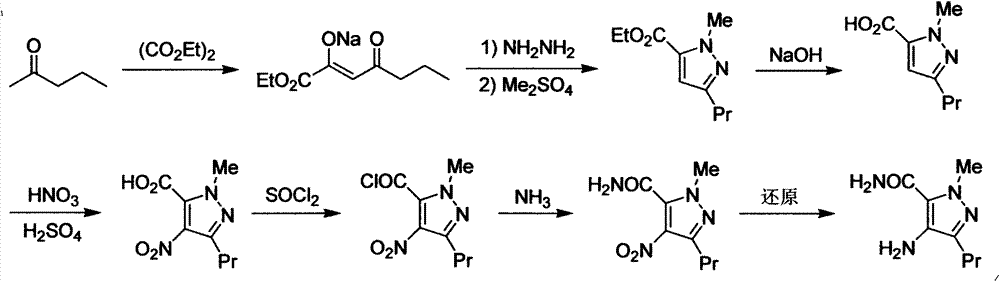
Novel method for preparing Sildenafil intermediate 4-amino-1-methyl-3-n-propyl-pyrazole-5-formamide
A technology of n-propyl pyrazole and formamide, which is applied in the field of pharmaceutical intermediate synthesis, can solve the problems of serious corrosion, a large amount of acid water, environmental protection pressure, etc., and achieves the effects of improving yield, avoiding strong corrosion and easier control of operation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: Preparation of ethyl 4-bromo-1-methyl-3-n-propylpyrazole-5-carboxylate (III)
[0023] Add 2500ml of dichloromethane and 196.1g of ethyl 1-methyl-3-n-propylpyrazole-5-carboxylate (1.0mol) into a 5000ml three-necked flask equipped with mechanical stirring, and control the reaction temperature below 10°C 239.8g (1.5mol) of liquid bromine was slowly added dropwise under dark conditions, and the dropwise addition was completed in about 1 hour, and the temperature was raised to room temperature for reaction, followed by TLC until the raw material point disappeared. Then slowly add 1000ml of 15% sodium carbonate solution dropwise, stir for 1h after the dropwise addition, let the layers stand, discard the water layer, dry the organic phase with anhydrous sodium sulfate, filter, evaporate the solvent, and wash the residue with ethyl acetate 261.4 g of the product was obtained by recrystallization, with a yield of 95.4%.
Embodiment 2
[0024] Embodiment 2: the preparation of 4-amino-1-methyl-3-n-propylpyrazole-5-carboxamide (I)
[0025] Add 200g of ethyl 4-bromo-1-methyl-3-n-propylpyrazole-5-carboxylate (0.73mol) and 1800ml of concentrated ammonia water into a 3000ml autoclave, close it, start stirring, and heat up to 60°C The reaction was followed by TLC until the raw material spots disappeared. After cooling down to room temperature, it was concentrated to dryness under reduced pressure, and the residue was recrystallized from ethyl acetate to obtain 140.8 g of the product, with a yield of 91%.
Embodiment 3
[0026] Embodiment 3: the preparation of 4-amino-1-methyl-3-n-propylpyrazole-5-carboxamide (I)
[0027] In a 3000ml autoclave, add 200g of 4-bromo-1-methyl-3-n-propylpyrazole-5-ethyl carboxylate (0.73mol), 1800ml of saturated ammonia ethanol solution and 57.7g of pyridine (0.73mol ), airtight, start stirring, heat up to 80°C for reaction, TLC tracking, until the raw material point disappears. After cooling down to room temperature, concentrate under reduced pressure to remove the solvent, dissolve the residue with 1500ml of dichloromethane, wash with 1N hydrochloric acid (600ml×3) and water (500ml×2) respectively, and recrystallize the residue with ethyl acetate to obtain the product 135.6g, yield 87.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com