Immune detection reagent for detecting respiratory syncytial virus
A syncytial virus and detection reagent technology, applied in the biological field, can solve the high-level problems of fluorescent quantitative PCR, achieve high sensitivity and specificity, and facilitate clinical promotion and use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
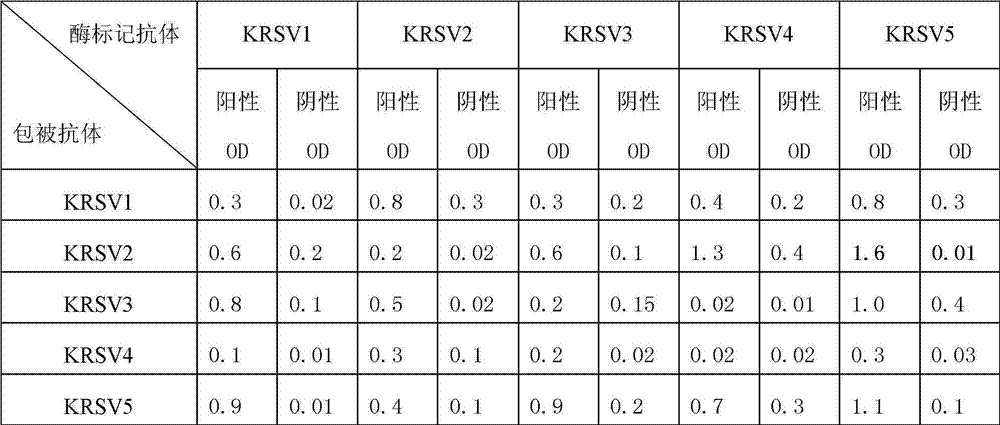
[0017] [Example 1] Development and paired screening of RSV monoclonal antibodies
[0018] 1. Preparation of Antigens for Immunization and Screening
[0019] In order to develop the RSV monoclonal antibody, from the proteomics analysis of the virus, the present invention selects the NP (nucleocapsid) protein, which is relatively conserved and relatively high in content, as the target protein for diagnosis.
[0020] 1.1 Express and purify the NP protein of RSV with Escherichia coli: the NP protein gene of RSV was expressed in Escherichia coli through PET30a plasmid, and the product was affinity-purified by HIS column chromatography, and used as an antigen for immunization. For the specific method and process, please refer to the literature (Sequence Analysis and Prokaryotic Expression of Regional Human Respiratory Syncytial Virus Nucleoprotein Gene, Journal of Virology, CHINESE JOURNAL OF VIROLOGY 2007.06.vol.23459-465).
[0021] 1.2 Cultivation and isolation and purification o...
Embodiment 2
[0048] [Example 2] Anti-respiratory syncytial virus (RSV) diagnostic kit
[0049] principle
[0050] This kit uses specific anti-RSV monoclonal antibody-coated microwell plate and biotin-labeled anti-RSV monoclonal antibody double-antibody sandwich method to detect the NP antigen of RSV virus, and then uses avidinase to amplify the reaction, which can improve the sensitivity and specificity Detection of RSV virus in throat swab extract.
[0051] Kit components (specifications) 96 samples:
[0052]
[0053]
[0054] Among them, clinically, because the detected sample is a patient's throat swab, the amount of virus contained is extremely low. In order to improve the clinical detection rate, the kit of the present invention adopts biotin-avidin amplification technology, that is, the BAS system (see "International Journal of Biological Products, No. 03, 1984 Authors: Li Shaokang; Zhang Gusheng.)
[0055] Preparation of pre-coated plate: Dissolve the purified KRSV2 ant...
Embodiment 3
[0085] [Example 3] Clinical trials of anti-respiratory syncytial virus (RSV) diagnostic kit
[0086] 1. Sensitivity experiment
[0087] The RSV fluorescent quantitative PCR reagent currently used clinically is used as a control reagent, and positive RSV virus culture (cell culture fluid) is taken as a sample to detect its sensitivity. RSV virus culture is diluted from 1600X to 51200X successively, and the OD value detected by ELISA method, its result is shown in the following table 2:
[0088] Table 2
[0089]
[0090] Judgment method according to the result of embodiment 2: reagent positive judgment value (CUTOFF)=2.1* negative control average OD value, negative value is less than 0.05 (calculated as 0.05), that is, OD value ≥ 0.1 is judged as positive, that is, the dilution corresponding to the sample sensitivity The degree is: 12800. The CT value corresponding to the fluorescent quantitative PCR of the sample 12800X is 28. This value is also the CUTOFF value of the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com