Ranitidine bismuth citrate intra-gastric floating sustained-release tablet and preparation method thereof
A technology of bismuth ranitidine citrate and gastric floatation, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as poor compliance and frequent medication by patients , to achieve good stability, long-lasting blood concentration, and reduce the number of medications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The preparation of embodiment 1RBC gastric floating tablet
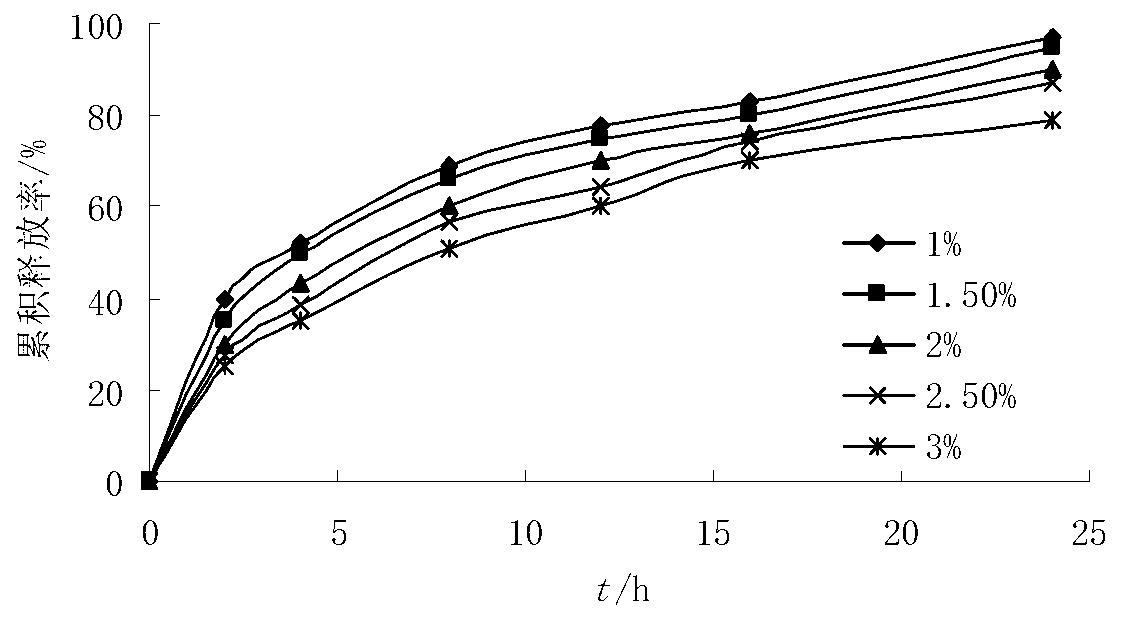
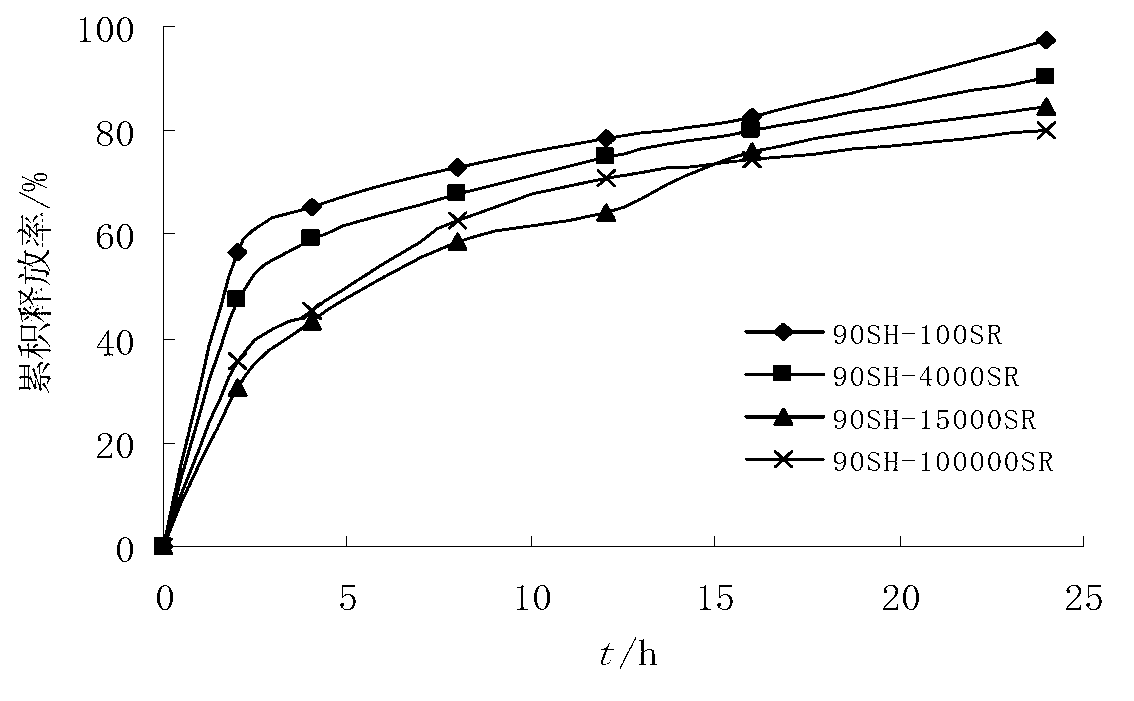
[0045] Tablet core formula: RBC raw material 228g, stearyl alcohol 240g, HPMC 15000SR 60g, HPMC 100000SR 30g, NaHCO 3 12g, micronized silica gel 2g, magnesium stearate 2g, absolute ethanol 10g.
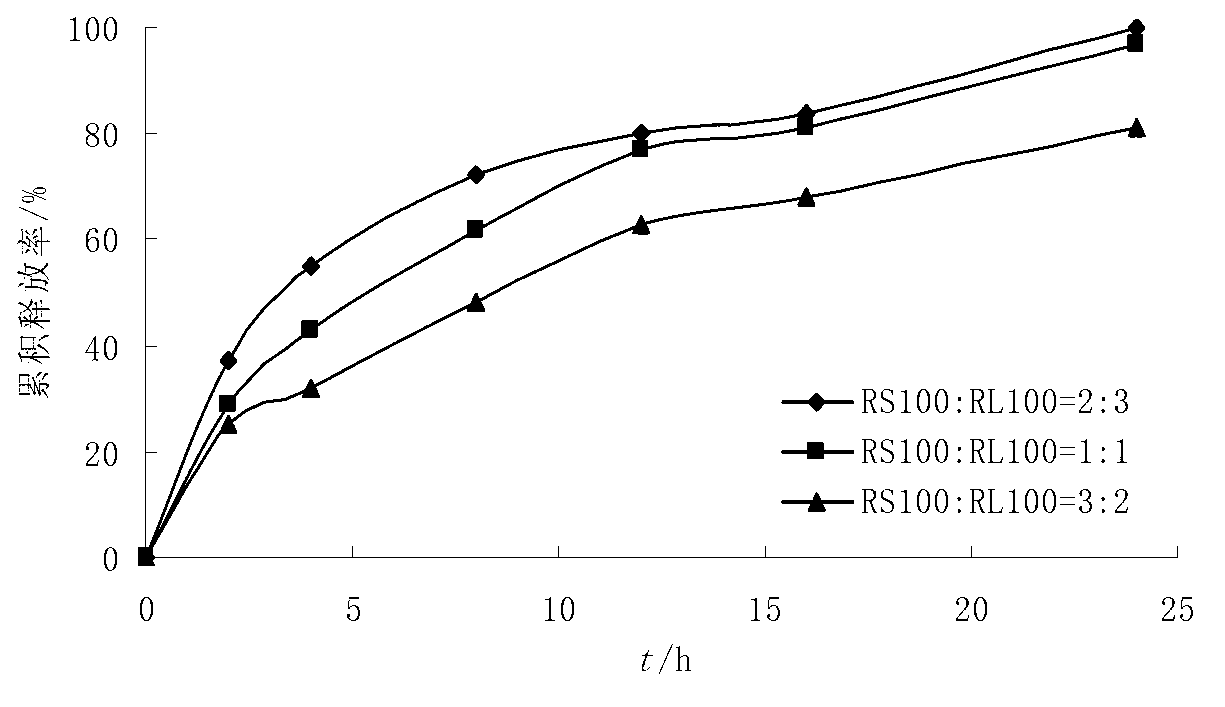
[0046] Coating film coating material: Eudragit RS100, RL100 30g each, triethyl citrate 6g, talcum powder 15g, ethanol 919g.
[0047] Preparation:
[0048] Pass the raw material and auxiliary materials through a 100-mesh sieve for later use; weigh the prescribed amount of raw materials and auxiliary materials in equal amounts and mix them uniformly, add an appropriate amount of binder, and make a soft material. Granulate through a 30-mesh sieve, and dry in an oven at 45°C for 2-3 hours. Sieve through a 24-mesh sieve, add a lubricant (the same amount of micro-powder silica gel and magnesium stearate) and mix well; control the tablet hardness at 40-50N, punch the tablet with a 12mm shallow concave; that is, the table...
Embodiment 2
[0051] The preparation of embodiment 2RBC gastric floating tablet
[0052] Tablet core formula: RBC raw material 228g, stearyl alcohol 240g, HPMC 15000SR 40g, HPMC 100000SR 20g, NaHCO 3 12g, magnesium stearate 4g, absolute ethanol 10g.
[0053] Coating film coating material: Eudragit RS100, RL100 30g each, triethyl citrate 6g, talcum powder 15g, ethanol 919g.
[0054] Preparation:
[0055] Pass the raw material and auxiliary materials through an 80-mesh sieve for later use; weigh the prescribed amount of raw materials and auxiliary materials in equal amounts and mix them uniformly, add an appropriate amount of binder, and make a soft material. Pass through a 30-mesh sieve to granulate, and dry in an oven at 50°C for 2 hours. Sieve with a 24-mesh sieve, add a lubricant and mix evenly; control the tablet hardness at 40-50N, punch the tablet with a 12mm shallow concave; that is, the tablet core.
[0056] Weigh the coating material in proportion and dissolve it in 500g of eth...
Embodiment 3
[0058] The preparation of embodiment 3RBC gastric floating tablet
[0059] Tablet core formula: RBC raw material 228g, stearyl alcohol 240g, HPMC 15000SR 80g, HPMC 100000SR 40g, NaHCO 3 12g, magnesium stearate 4g, absolute ethanol 10g.
[0060] Coating film coating material: Eudragit RS100, RL100 30g each, triethyl citrate 6g, talcum powder 15g, ethanol 919g.
[0061] Preparation:
[0062] Pass the raw material and auxiliary materials through a 120-mesh sieve for later use; weigh the prescribed amount of raw materials and auxiliary materials in equal amounts and mix them uniformly, add an appropriate amount of binder, and make a soft material. Granulate through a 30-mesh sieve, and dry in an oven at 40°C for 3 hours. Sieve with a 24-mesh sieve, add a lubricant and mix evenly; control the tablet hardness at 40-50N, punch the tablet with a 12mm shallow concave; that is, the tablet core.
[0063] Weigh the coating material in proportion and dissolve it in 500g of ethanol, ad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com