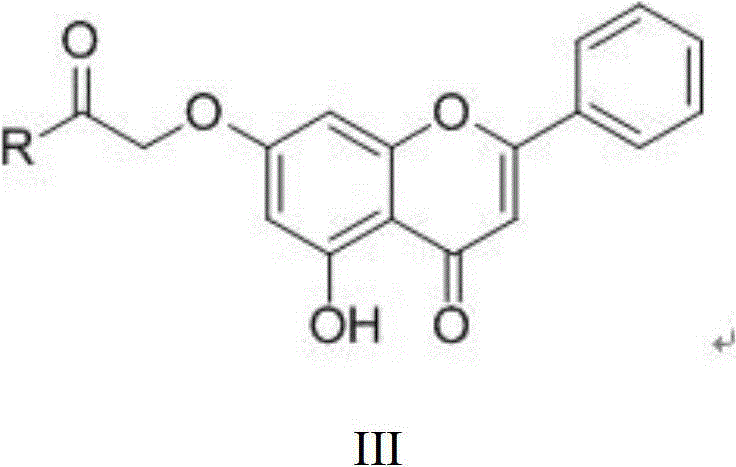
Chrysin amide derivative and medical application thereof
A pharmacy and compound technology, applied in the field of chrysinamide derivatives and their medicinal uses, can solve the problems that the research of chrysinamide derivatives has not yet been reported, and achieve the effects of excellent antitumor drugs, inhibition of sarcoma size, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
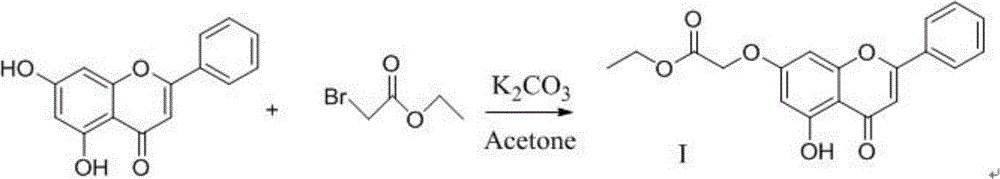
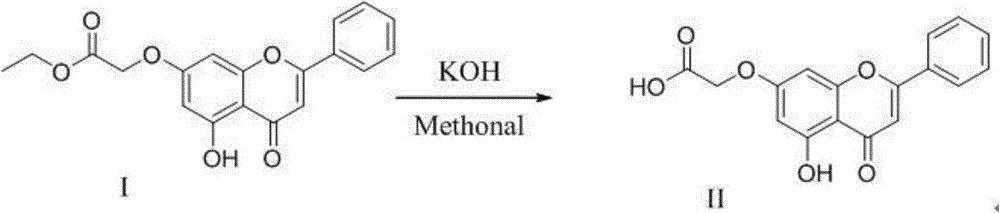
[0020] N-(1-ethylpyrrolidine-2-methyl)-2-(5-hydroxyl-4-oxygen-2-phenyl-4H-benzopyrone-7-oxyl)acetamide preparation (1 ), the preparation of ethyl 2-(5-hydroxy-2-phenyl-4H-benzopyrone-7-oxo)acetate
[0021]
[0022] Chrysin (2.54g; 0.01mol) and anhydrous potassium carbonate (1.66g; 0.011mol) were added to 100ml of stirred acetone, then ethyl bromoacetate (1.84g, 0.011mol) was added to the solution, and the reaction was Heating to reflux was carried out under the condition of heating (50~60° C.) while stirring, and the reaction time was 6 hours. After the reaction was over, it was cooled. The solution was suction-filtered, and the filtrate was concentrated to 40% of the original volume after suction filtration. The filtrate concentrate was washed with petroleum ether, 1 wt% NaOH solution and water in turn, and then vacuum-dried. The product is a light yellow solid, the product yield is 91%, mp: 242-244°C, 1 H NMR (400MHz, d 6 -DMSO):1.25(t,J=6.9Hz,3H);4.19(m,2H);4.96(S,2H...
Embodiment 2
[0030] 5-Hydroxy-7-(2-oxo-2-piperazinylethoxy)-2-phenyl-4H-benzopyrone
[0031]
[0032] A mixture of dicyclohexylcarbodiimide (DCC, 0.75g; 3.372mmol) and 20ml of anhydrous dichloromethane was dripped into 40ml of anhydrous dichloromethane obtained in step (2) of Example 1 (0.96g; 3.06mmol). In the methyl chloride solution, the whole process was carried out in an ice bath, and after the dropwise addition was completed, the reaction was carried out in an ice bath for 12 hours. Remove the ice bath, add the compound piperazine 3.373mmol and triethylamine TEA (0.341g; 3.372mmol), stir at room temperature for 12 hours, filter with suction, concentrate the filtrate to dryness, then add 80ml of ethyl acetate, and filter with suction , the filtrate was washed 3 times with dilute potassium carbonate solution (concentration 1wt%) and saturated NaCl solution respectively, and the organic phase was washed 3 times with dilute HCl (0.30~0.40wt%), and the aqueous phase was combined, and t...
Embodiment 3
[0034] 2-(5-Hydroxy-4-oxo-2-phenyl-4H-benzopyrone-7-oxyl)-N-(2-piperidinylethyl)acetamide
[0035]
[0036] A mixture of dicyclohexylcarbodiimide (DCC, 0.75g; 3.372mmol) and 20ml of anhydrous dichloromethane was dripped into 40ml of anhydrous dichloromethane obtained in step (2) of Example 1 (0.96g; 3.06mmol). In the methyl chloride solution, the whole process was carried out in an ice bath, and after the dropwise addition was completed, the reaction was carried out in an ice bath for 12 hours. Remove the ice bath, add compound 1-(2-aminoethyl)piperidine 3.373mmol and triethylamine TEA (0.341g; 3.372mmol), stir at room temperature for 12 hours, filter with suction, and concentrate the filtrate to dryness. Then add 100ml of ethyl acetate, filter with suction, wash the filtrate with dilute potassium carbonate solution (concentration 1wt%) and saturated NaCl solution 3 times respectively, wash the organic phase with dilute HCl (0.30~0.40wt%) 3 times, and combine the water phas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com