Therapeutic agent for disease
A disease treatment, nanocapsule technology, applied in metabolic diseases, nervous system diseases, cardiovascular system diseases, etc., can solve the problems that cannot be used as medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0115] Example 1 (terminal cancer)
[0116] 1) animals
[0117] As animals, five 6-week-old C57BL / 6Cr Slc female mice were used for each group.
[0118] 2) Reagent
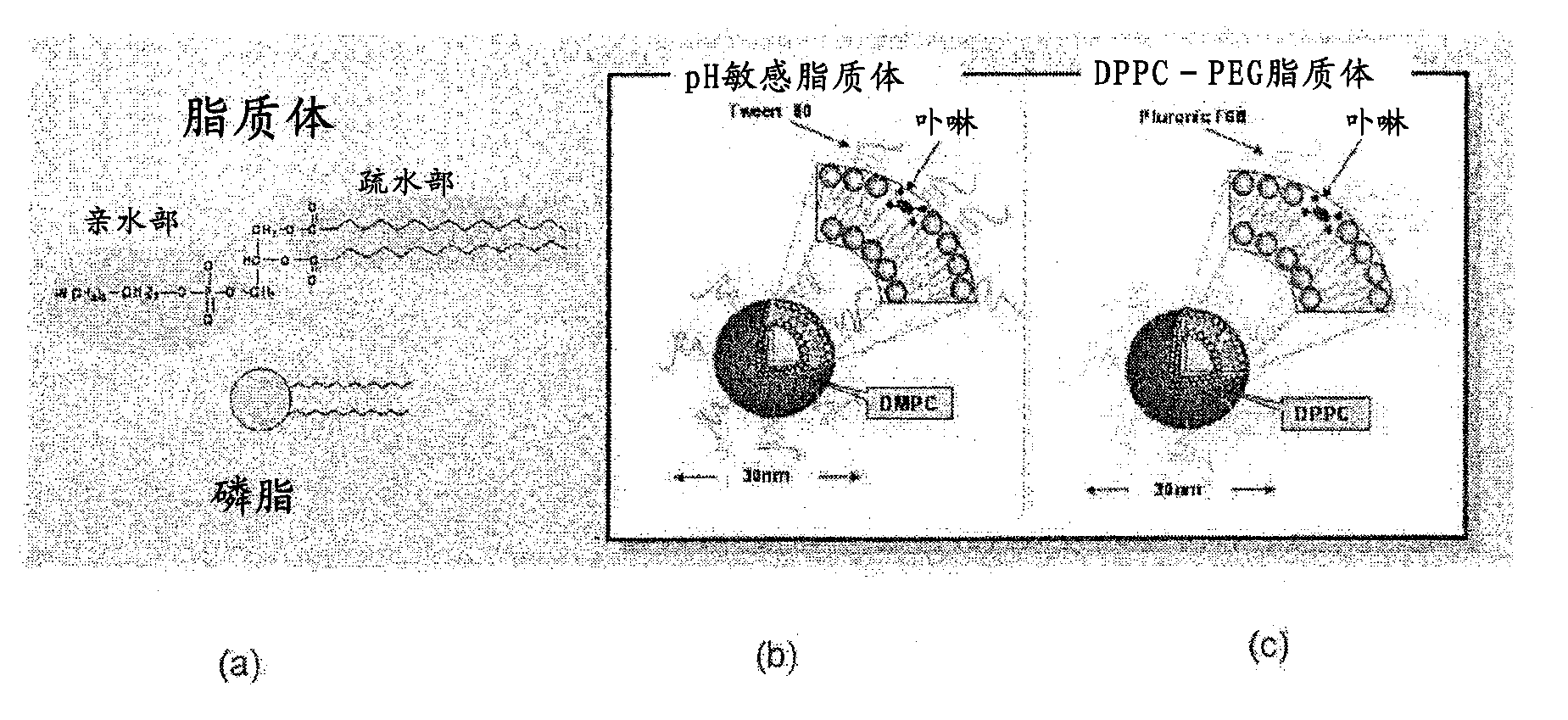
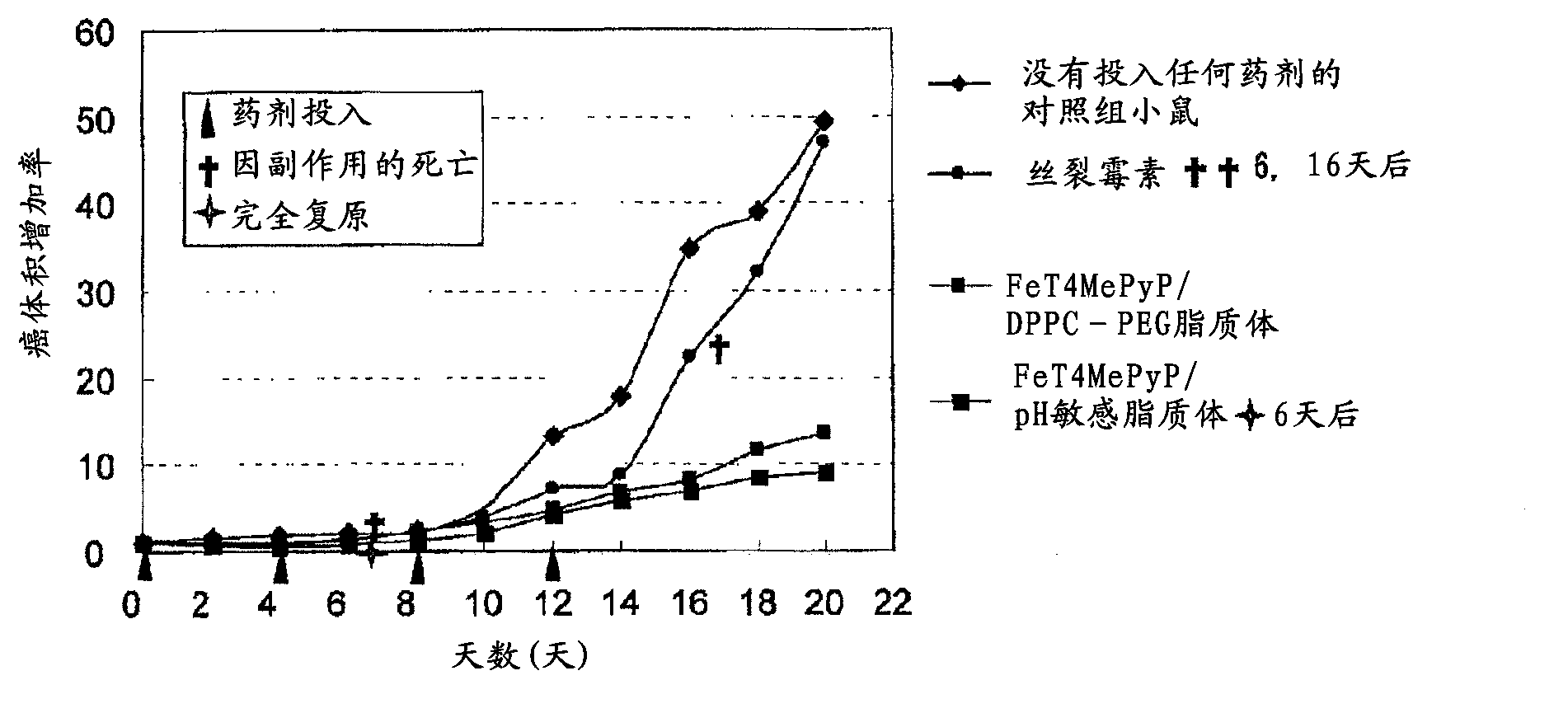
[0119] As the reagent (concentration) of the present invention, iron porphyrin complex / pH-sensitive liposome (5 mM / 36 mM) and iron porphyrin complex / DPPC-PEG liposome (5 mM / 36 mM) were used.
[0120] As the reagent (concentration) of the comparative example, mitomycin C (MMC) (0.9 mM) was used.
[0121] 3) Cancer cells
[0122] B16 melanoma cells were used.
[0123] 4) Test method
[0124] B16melanoma dispersed in PBS was injected into the footpad (sole) of a mouse (C57BL / 6, ♀, 6 weeks old) to transplant cancer. The input amount of cancer cells is 1×10 6 pcs / piece / 0.05ml. An environment where a large number of thin blood vessels such as capillaries exist is selected within the range limited to the foot pads as the place for injection.
[0125] Adherent cancer was confirmed on the 10th day after cancer cel...
Embodiment 2
[0142] Example 2 (early cancer)
[0143] 1) animals
[0144] As animals, five 6-week-old ICR female mice were used for each group.
[0145] 2) Reagent
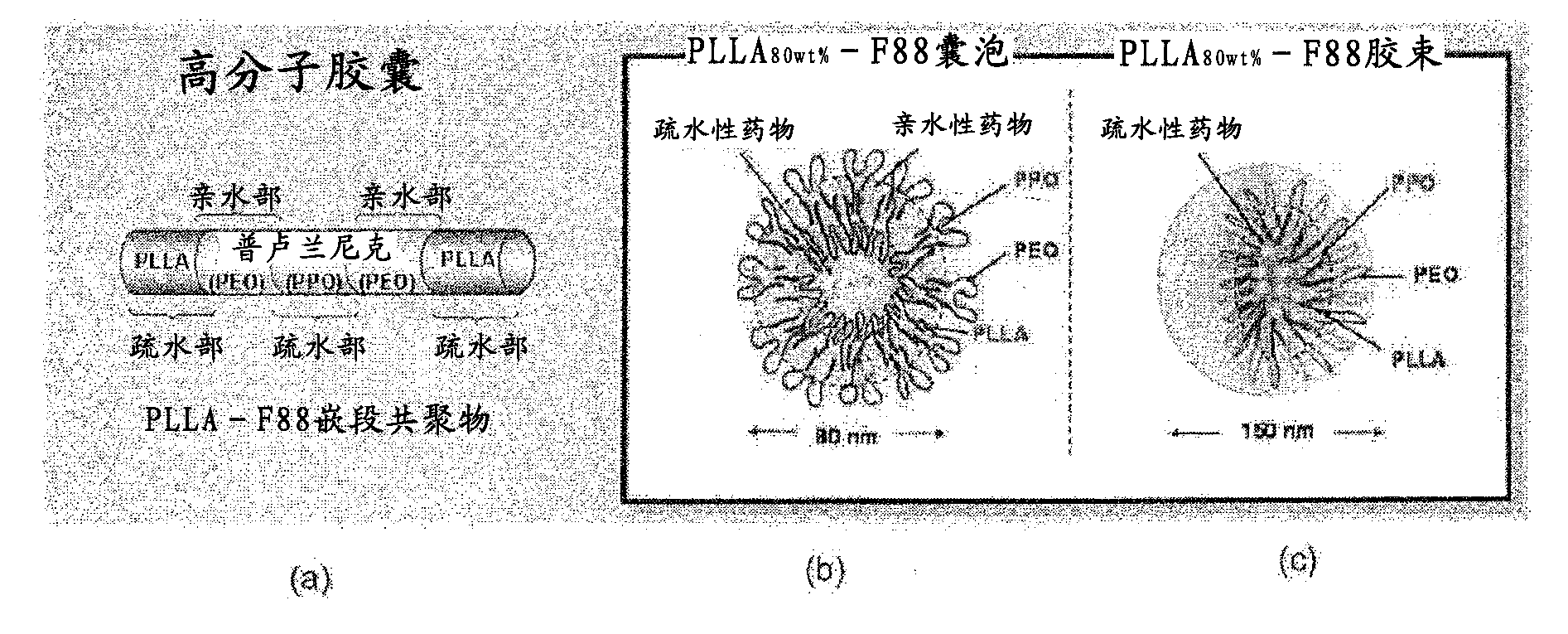
[0146] As the reagent (concentration) of the present invention, iron porphyrin complex / PLLA was used 60wt% - F88 micelles (5mM / 0.7wt%).
[0147] As the reagent (concentration) of the comparative example, cisplatin (CDDP) (0.9 mM) was used.
[0148] 3) Cancer cells
[0149] B16 melanoma cells were used.
[0150] 4) Test method
[0151] B16melanoma dispersed in PBS was injected into the footpad (sole) of a mouse (C57BL / 6, ♀, 6 weeks old) to transplant cancer. The input amount of cancer cells is 1 / 10 of the terminal cancer situation, that is, 1×10 5 pcs / piece / 0.05ml. An environment where a large number of thin blood vessels such as capillaries exist is selected within the range limited to the foot pads as the place for injection.
[0152] Adherent cancer was confirmed on the 10th day after cancer cell transplantation, a...
Embodiment 3
[0164] Example 3 (kidney disease)
[0165] 1) animals
[0166] As animals, 6-week-old HIGA / Nsc Slc female mice were used. As for the mice in the control group, HIGA mice were inbred and derived from ddY mice, and BALB / C mice were used according to the experimental instructions of SLC in Japan.
[0167] 2) Reagent
[0168] As the reagent (concentration) of the present invention, use
[0169] Manganese porphyrin complex / pH sensitive liposome (5mM / 36mM),
[0170] Manganese porphyrin complex / DPPC-PEG liposome (5mM / 36mM),
[0171] Manganese porphyrin complex / PLLA 80wt% - F88 vesicles (100 μΜ / 0.7 wt%).
[0172] 3) Test method
[0173] Mice (HIGA / Nsc Slc, ♀, 6 weeks old) were administered 0.2ml of the drug into the tail vein every week, and urine protein and occult blood were checked once a week through a urine test, and observed for one month. In addition, for the urinalysis drug, prediction 10II (Wako Pure Chemical Industries) was used.
[0174] 4) Results
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com