Method for preparing supported metal catalyst with high dispersion active center
A metal catalyst and supported technology, which is applied in the field of preparation of supported metal catalysts, can solve the problems of high catalyst production cost, unfavorable large-scale application, low reaction temperature, etc., and achieve simple and reliable preparation process, increased dispersion, The effect of using less
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Effect of Metal Loading
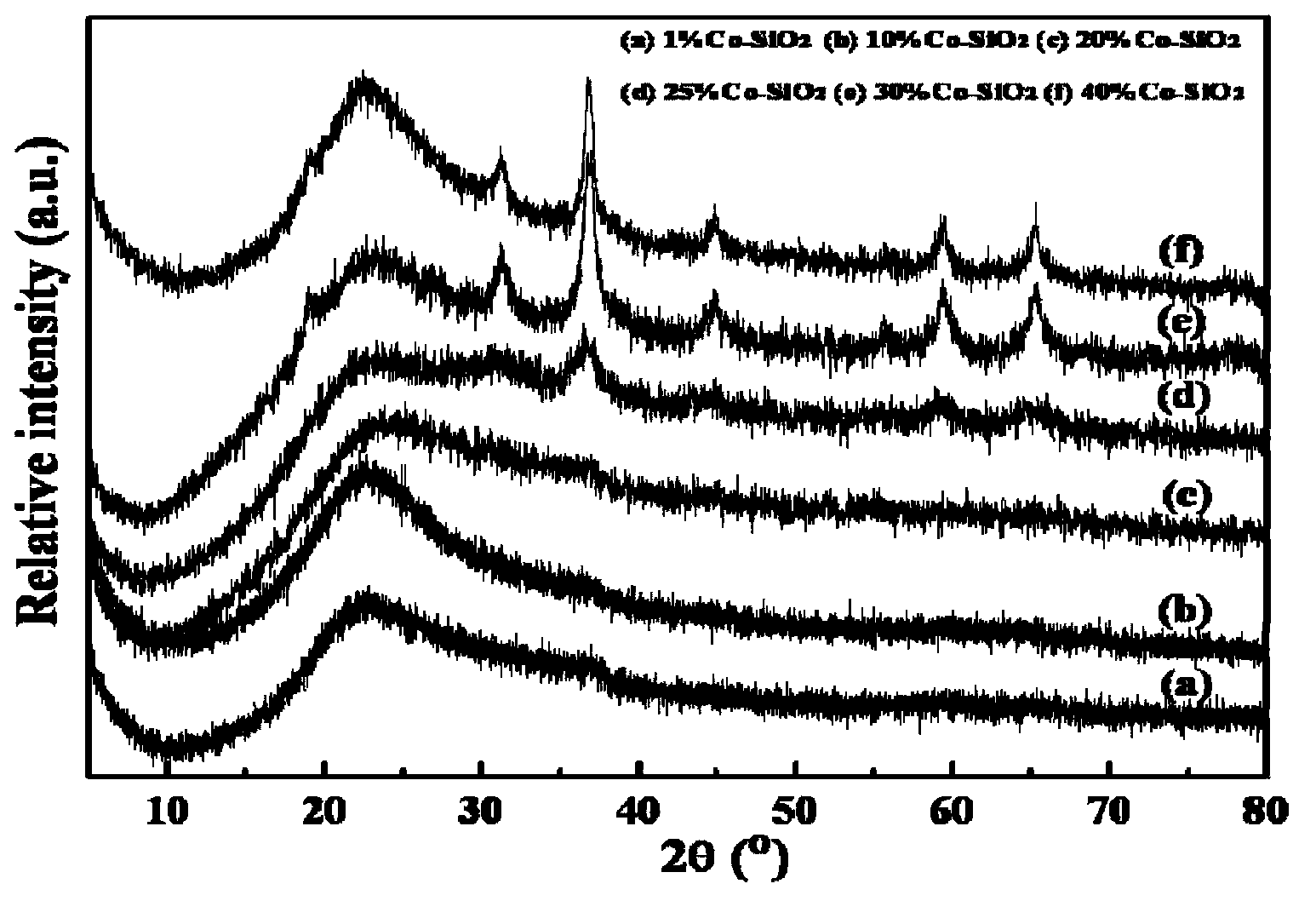
[0026] Weigh 2.5g granular 40-60 mesh commercial carrier SiO 2 Dry at 120°C for 12 hours and set aside. At room temperature, add ethylene glycol (glycol, abbreviated as EG) to impregnate SiO 2 The carrier was dried for 6 hours, and then dried at 50° C. for 24 hours to obtain a surface-modified carrier. At room temperature, 2.4692g Co(NO 3 ) 2 ·6H 2 O aqueous solution, soaked for 12 hours, 90°C spin-dried for 12 hours, 120°C dried for 12 hours, dried samples were placed in a muffle furnace at 400°C for 2 hours, the heating rate was 2°C / min, and 10% loaded Co / SiO 2 Catalyst, changing Co(NO 3 ) 2 ·6H 2 The amount of O, repeat the above process, the resulting catalyst is recorded as 1%Co-SiO 2 , 10%Co-SiO 2 , 20%Co-SiO 2 , 25%Co-SiO 2 , 30%Co-SiO 2 , 40%Co-SiO 2 . For the corresponding XRD spectrum, see figure 1 , it can be concluded from the figure that when the metal loading is below 25wt%, the metal active center is ...
Embodiment 2
[0027] Embodiment 2: the influence of sintering temperature
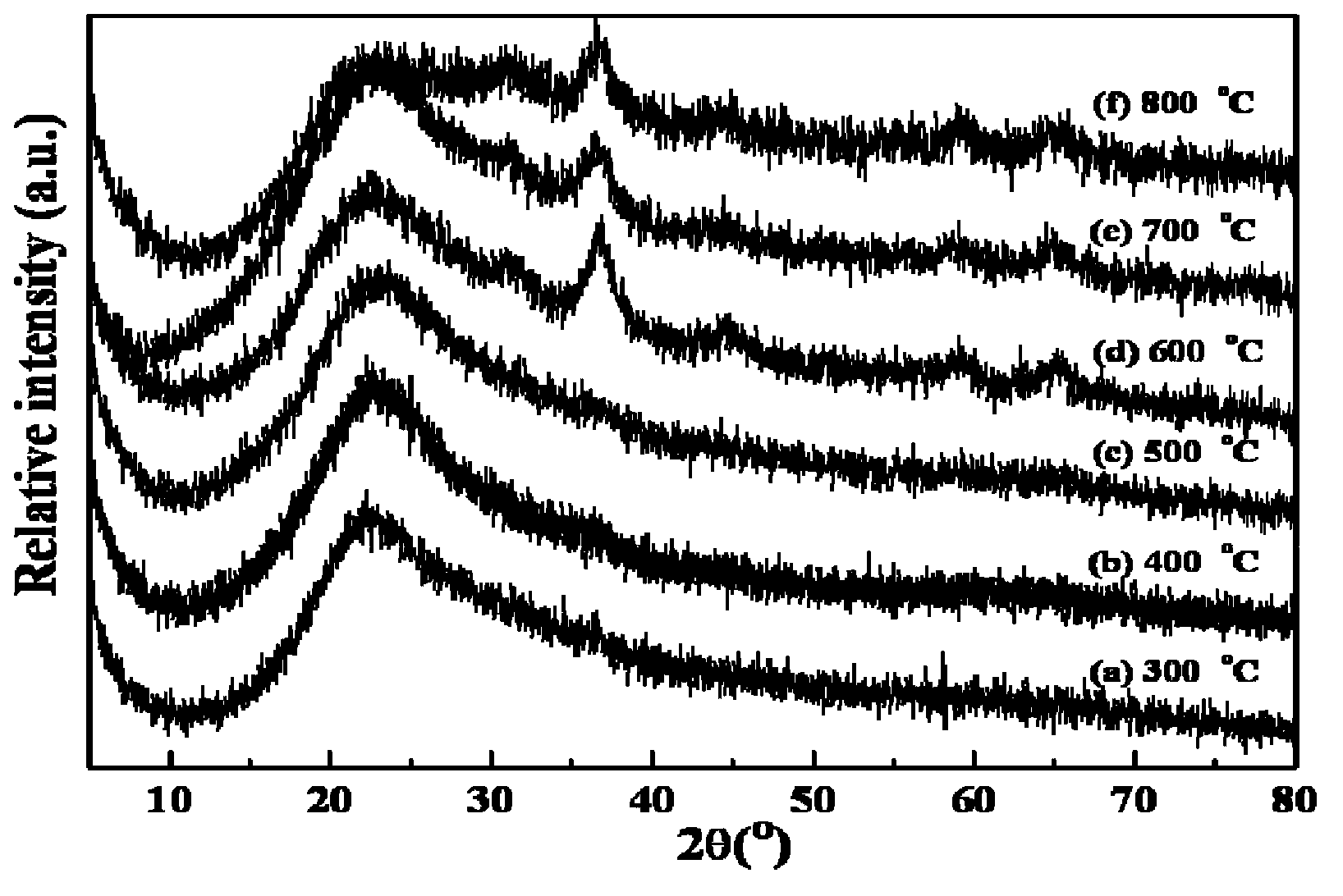
[0028] Implementation steps and conditions are the same as embodiment 1, commercial carrier SiO 2 Dry at 100°C for 24 hours with 10% Co / SiO loading 2 Catalyst as an example. At room temperature, add ethylene glycol (EG) to impregnate SiO 2 The carrier was dried for 24 hours, and then dried at 150° C. for 6 hours to obtain a surface-modified carrier. Co(NO 3 ) 2 ·6H 2 Immerse after O for 20 hours, spin dry at 50°C for 24 hours, and dry at 200°C for 10 hours. After drying, the sample is placed in a muffle furnace and roasted for 2 hours at 300°C, 400°C, 500°C, 600°C, 700°C, and 800°C. Rate 2°C / min. For the corresponding XRD spectrum, see figure 2 , the active center of the sample whose calcination temperature is below 600℃ is on the carrier SiO 2The loading is very uniform, the dispersion is very high, and the XRD diffraction peak completely disappears; at a calcination temperature above 600°C, a very diffus...
Embodiment 3
[0029] Embodiment 3: the influence of ethylene glycol dosage
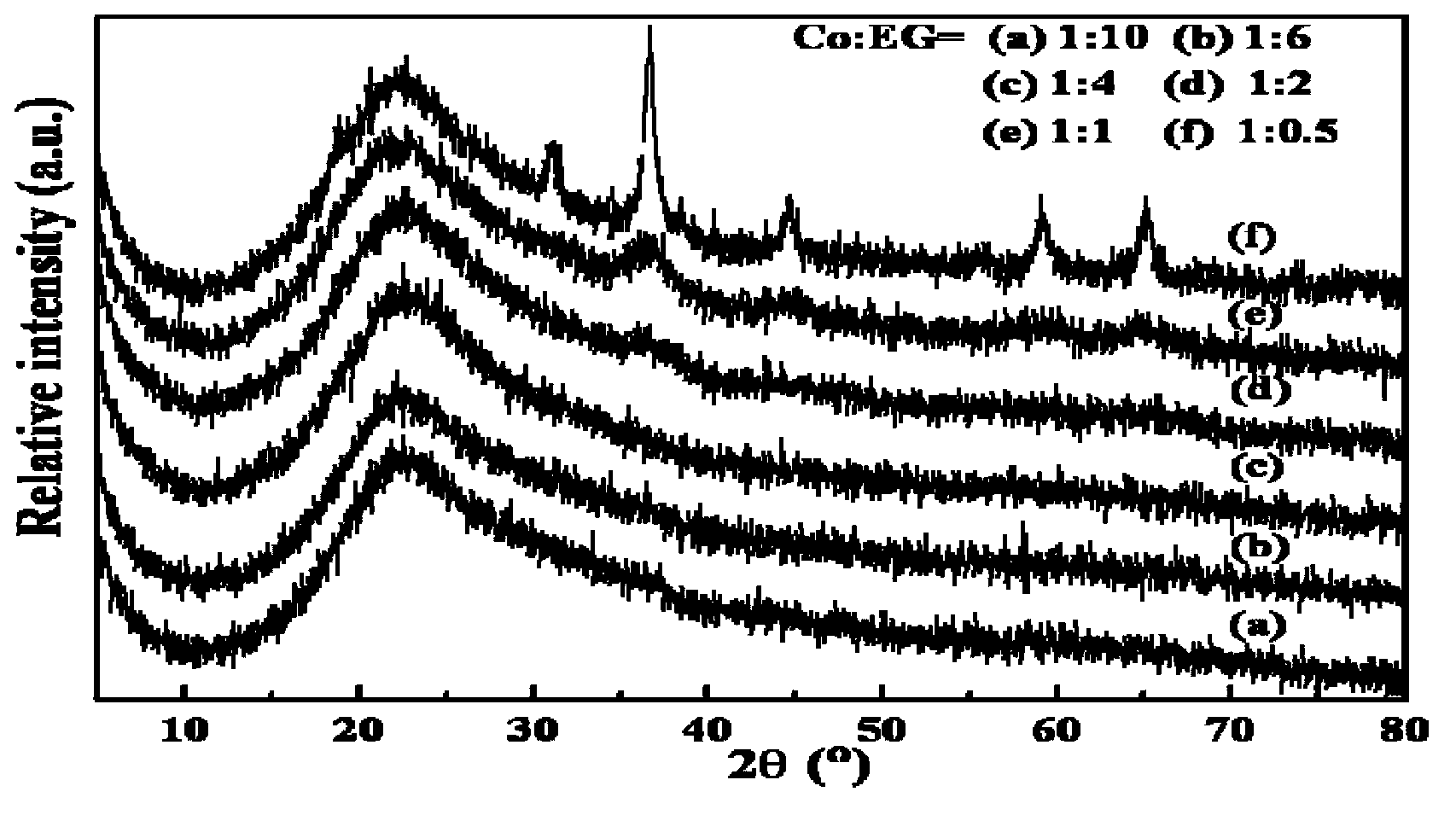
[0030] Weigh 2.5g granular 40-60 mesh commercial carrier SiO 2 Dry at 200°C for 6 hours and set aside. At room temperature, 2.4692g Co(NO 3 ) 2 ·6H 2 O in ethylene glycol aqueous solution, soaked for 6 hours, spin-dried at 60°C for 20h, and dried at 100°C for 24 hours; after drying, the sample was placed in a muffle furnace and calcined at 400°C for 2 hours at a heating rate of 2°C / min to obtain 10 % load Co / SiO 2 Catalyst; wherein the molar ratios of the metal in the metal salt to ethylene glycol are: 1:0.5, 1:1, 1:2, 1:4, 1:6, 1:10. For the corresponding XRD spectrum, see image 3 , it can be concluded from the figure that when the molar ratio of metal to ethylene glycol in the metal salt solution is less than 1:1, the metal active center is on the carrier SiO 2 The load is very uniform, the dispersion is very high, and the XRD diffraction peak completely disappears; when the ratio is 1:1, a very diffuse X...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com