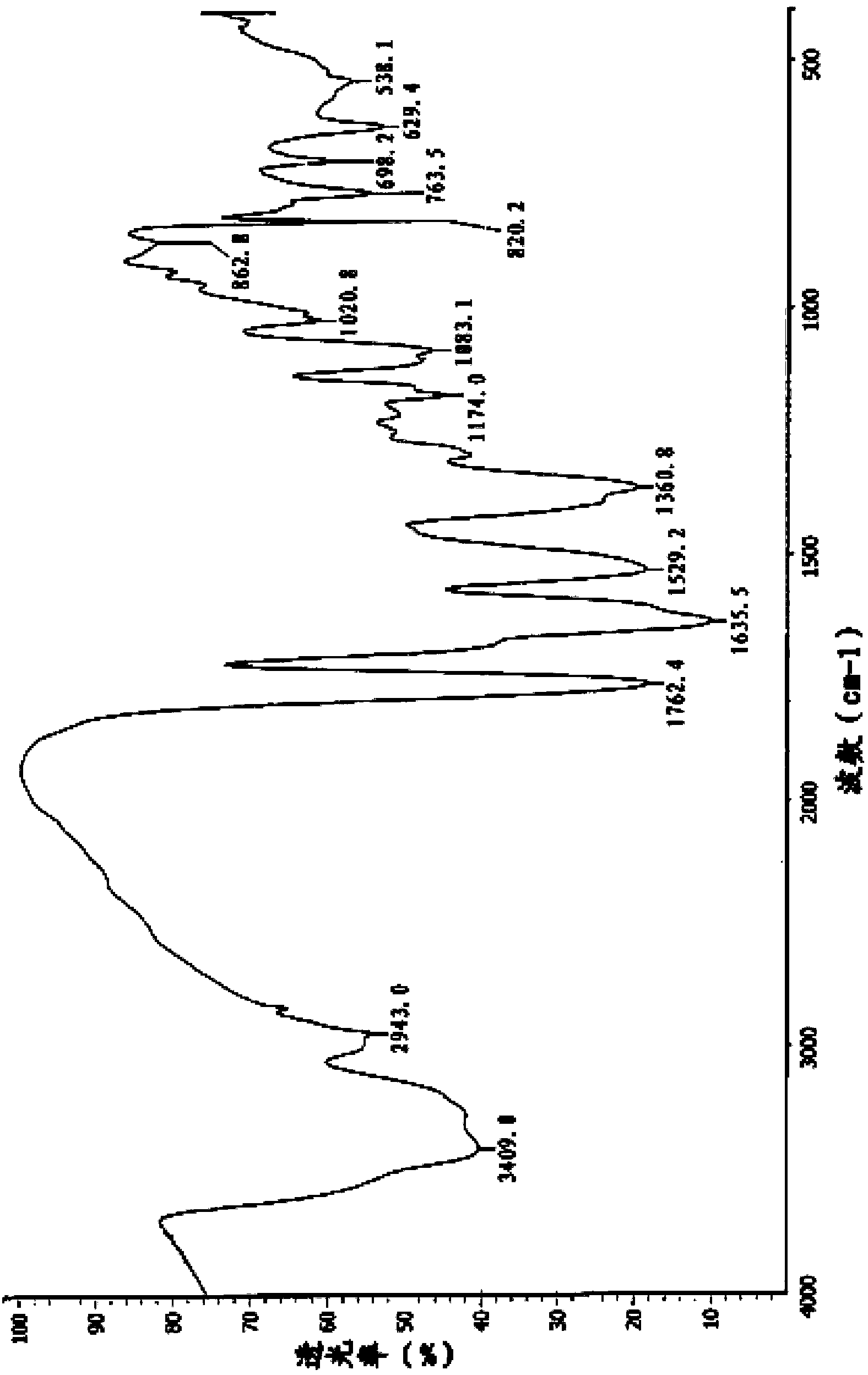
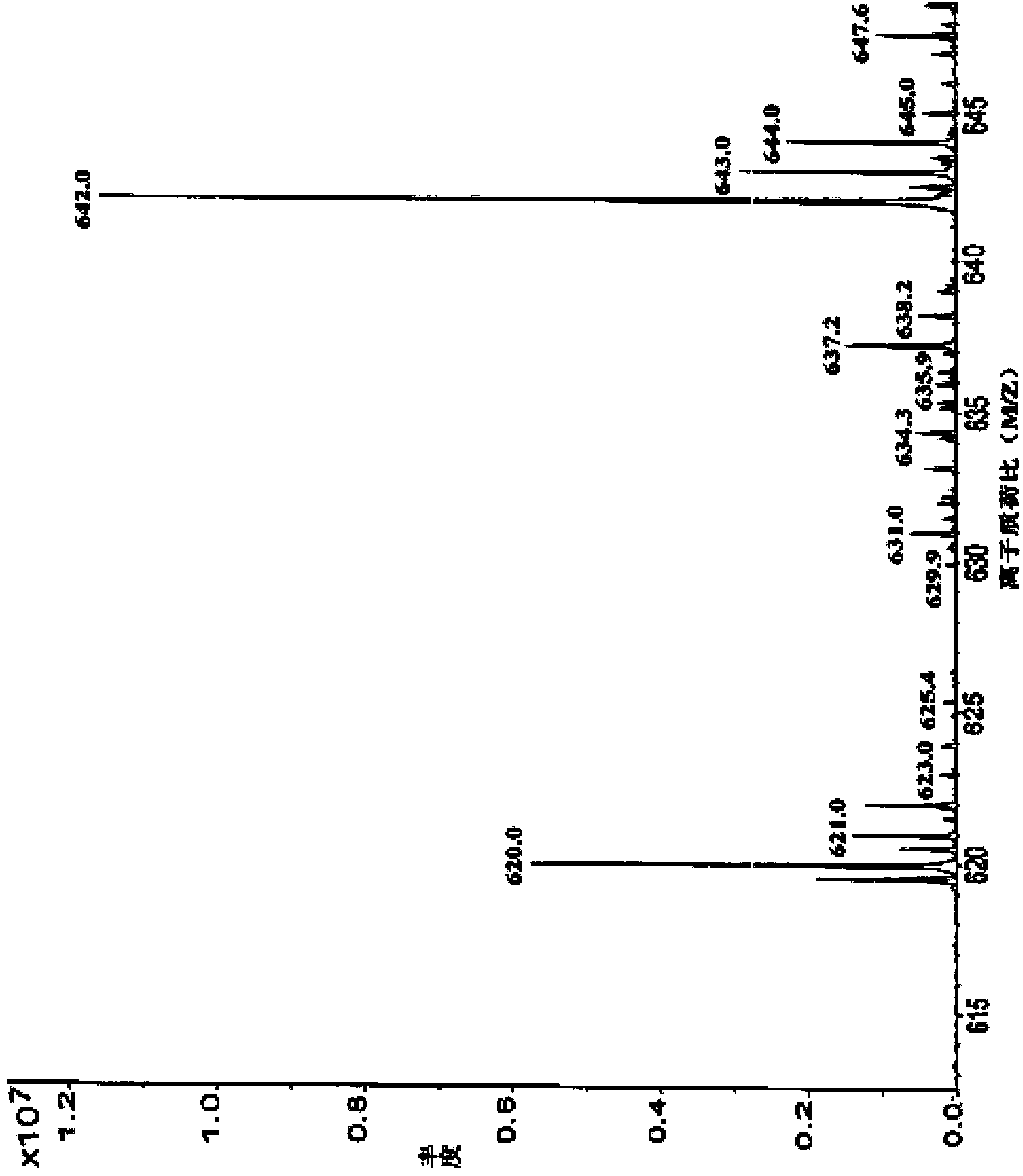
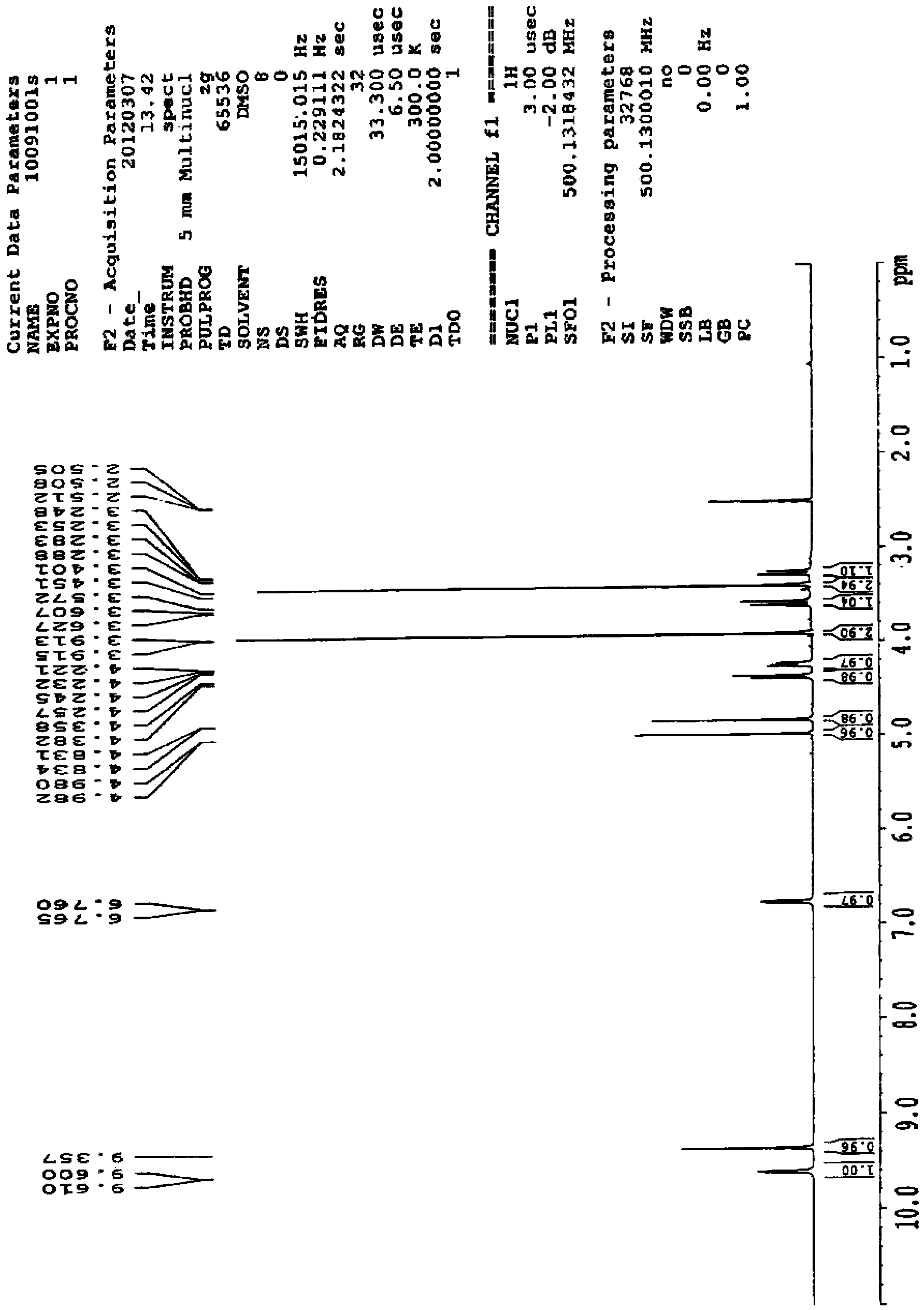
Preparation method for cefotetan disodium
A technology of cefotetan disodium and cefotetan acid, which is applied in the field of medicine, can solve problems such as poor clarity, dark product color, and solvent residue content, and achieve easy raw materials, low content, and polymer and related substance content low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] The present invention provides a method for preparing cefotetan disodium, and those skilled in the art can learn from the content of this article and appropriately improve the process parameters. In particular, it should be pointed out that all similar substitutions and modifications are obvious to those skilled in the art, and they are all deemed to be included in the present invention. The method and application of the present invention have been described through the preferred embodiments. It is obvious that relevant persons can modify or appropriately change and combine the methods and applications described herein without departing from the content, spirit and scope of the present invention to achieve and Apply the technology of the present invention.
[0056] The present invention provides a method for preparing cefotetan disodium. First, take cefotetan acid to form a first salt and mix it with silica gel, collect the first filtrate through the first filtration, and ...
Embodiment 1
[0080] Example 1 Preparation of Cefotetan Acid
[0081] 30.00kg of 7β-amino-7α-methoxy-3-[(1-methyl-1H-tetrazol-5-yl)thiomethyl]-3-cephem-4-carboxylic acid benzhydryl (7-MAC) was added to a 500L enamel reactor filled with 180L of dichloromethane, stirred for 30 minutes to dissolve, cooled to -20°C, added 10.5kg of N,N-dimethylaniline and 16.5kg of bromoacetyl bromide, After reacting for 1 hour, the reaction solution was washed with 30L ice water, 30L saturated potassium hydrogen sulfate solution, 30L saturated sodium bicarbonate solution (stirring for 30 minutes each time, standing for 30 minutes), and layered, the methylene chloride layer was washed with 2kg After drying with sodium sulfate for 60 minutes, the filtrate was dried under reduced pressure at 40°C, and the solvent was recovered to obtain 25.2kg of residual oil, which is 7β-bromoacetamido-7α-methoxy-3-(1-methyl-1H) -5-tetrazolyl)thiomethyl-3-cephem-4-carboxylic acid benzhydryl;
[0082] Take 25kg of 7β-bromoacetamido-...
Embodiment 2
[0084] Example 2 Preparation of Cefotetan Acid
[0085] 30.00kg of 7β-amino-7α-methoxy-3-[(1-methyl-1H-tetrazol-5-yl)thiomethyl]-3-cephem-4-carboxylic acid benzhydryl (7-MAC) was added to a 500L enamel reactor filled with 300L of dichloromethane, stirred for 60 minutes to dissolve, cooled to -30°C, added 13.5kg of N,N-dimethylaniline and 21.0kg of bromoacetyl bromide, After reacting for 1 hour, the reaction solution was washed with 60L ice water, 60L saturated potassium hydrogen sulfate solution, 60L saturated sodium bicarbonate solution in sequence (stirring for 30 minutes each time, standing for 30 minutes), layering, the dichloromethane layer with 4kg After drying with sodium sulfate for 60 minutes, the filtrate was dried under reduced pressure at 40°C, and the solvent was recovered to obtain 25.4 kg of residual oil, which is 7β-bromoacetamido-7α-methoxy-3-(1-methyl-1H) -5-tetrazolyl)thiomethyl-3-cephem-4-carboxylic acid benzhydryl;
[0086] Take 25kg of 7β-bromoacetamido-7α-m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com