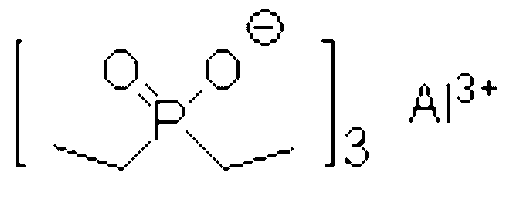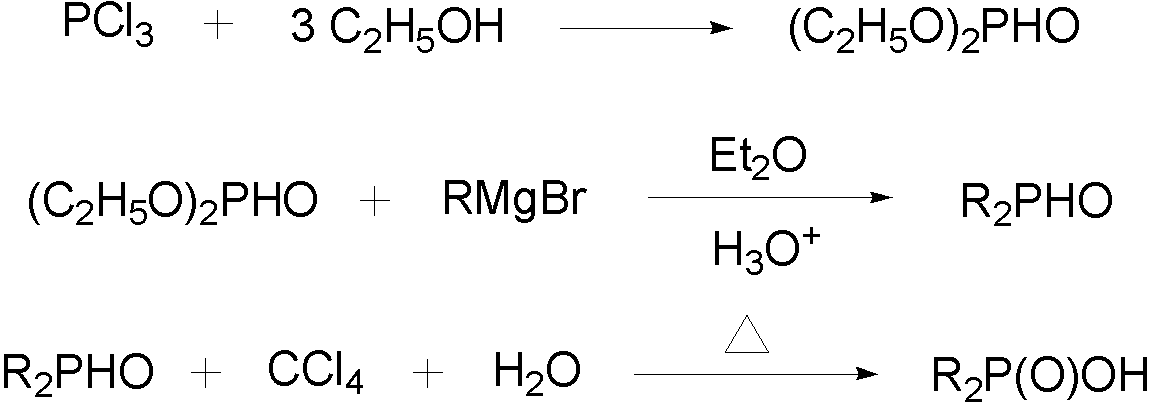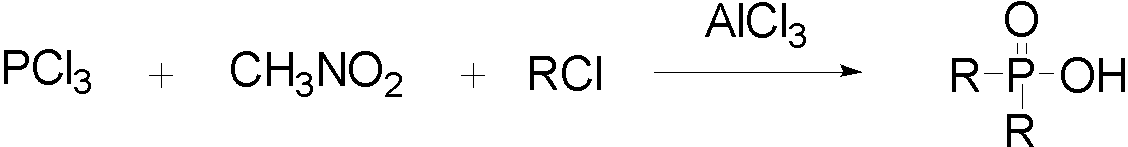Dibenzyl phosphinic acid synthetic method
A technology of dibenzylphosphinic acid and dibenzyl, applied in the field of synthesizing dibenzylphosphinic acid, can solve the problems of low product purity, complex raw materials, low yield, etc., achieve large extraction capacity and reduce side reactions , the effect of increasing the reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Put 1.32 g (0.055 mol) magnesium bar in a 250 mL three-necked flask equipped with stirring, reflux condenser and dropping funnel, immerse the magnesium bar with a small amount of anhydrous ether, and add a small grain of iodine. Add 5.8 mL (0.05 mol) of benzyl chloride and 24 mL of anhydrous ether to the dropping funnel and mix well. The oil bath was heated to about 40-50 °C, and about 1 / 10 of the total amount (about 3 ml) of benzyl chloride anhydrous ether solution was slowly added dropwise. When the temperature of the system rises and starts to reflux, start stirring, slowly add the remaining benzyl chloride solution, control the dripping speed to keep the reaction liquid in a slightly boiling state, and after the dripping is completed, wait for the reaction to stop, cool the reaction bottle slightly, and start the dripping. Slowly add a mixture of 2.8 mL (0.022 mol) diethyl phosphite and 30 mL of anhydrous ether into the liquid funnel (control the dripping speed and ...
Embodiment 2
[0060] Put 9.3 g (0.387 mol) magnesium bar in a 250 mL three-necked flask equipped with stirring, reflux condenser and dropping funnel, immerse the magnesium bar with a small amount of anhydrous ether, and add a small grain of iodine. Add 40.5 mL (0.352 mol) of benzyl chloride and 96 mL of anhydrous ether to the dropping funnel and mix well. The oil bath was heated to about 40-50 °C, and about 1 / 10 of the total amount (about 15 ml) of benzyl chloride anhydrous ether solution was slowly added dropwise. When the temperature of the system rises and starts to reflux, start stirring, slowly add the remaining benzyl chloride solution, control the dripping speed to keep the reaction liquid in a slightly boiling state, and after the dripping is completed, wait for the reaction to stop, cool the reaction bottle slightly, and start the dripping. Slowly add a mixture of 11.3 mL (0.088 mol) diethyl phosphite and 120 mL anhydrous ether into the liquid funnel (control the dripping speed and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com