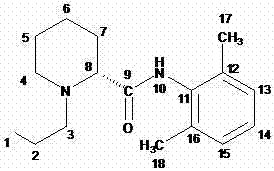
Method for preparing ropivacaine
A piperidine and direct technology, which is applied in the field of preparation of local anesthetic ropivacaine, can solve the problems of being dangerous and unsuitable for industrial production, and achieve the effects of simple operation, increased yield and reduced cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
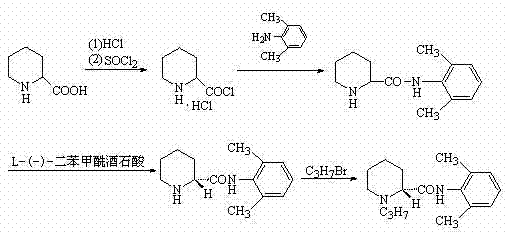
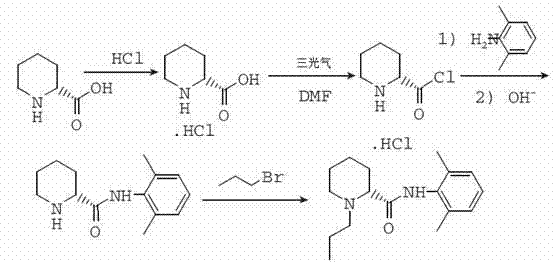
[0029] Mix 6.5g of L-piperidine-2-carboxylic acid with 100ml of toluene, and slowly inject HCl gas under stirring at room temperature until the pH of the reaction solution is 3. When the reaction solution is heated to 40°C, add 0.1ml of DMF, and dropwise add 6g of oxychloride at 40°C. Sulfone, after dropping, keep stirring at the temperature for 3 hours, add dropwise 20ml of toluene solution containing 12.1g 2,6-dimethylaniline, the reaction is exothermic, control the internal temperature not to exceed 60°C, add 7.3g bromopropane after 2 hours of reaction , 4.5gK 2 CO 3 , reacted at 50°C for 4 hours under stirring, filtered hot, and placed the filtrate at 0°C for crystallization. It was filtered and sucked dry, and dried to obtain 12 g of white solid.
[0030] Refining: heat-dissolve the crude product in 50ml of toluene, filter, cool, crystallize and filter to obtain 10g of fine product, with a total yield of 71%.
[0031] ES-MS m / z 275 [M+H + ], mp: 143~146°C, [α]25 D -80...
Embodiment 2
[0038] Mix 6.5g of L-piperidine-2-carboxylic acid with 100ml of toluene, slowly inject HCl gas under stirring at room temperature until the pH of the reaction solution is 3, heat the reaction solution to reflux, add 0.1ml of DMF, and add 6g of thionyl chloride dropwise while maintaining the reflux temperature, After dropping, keep stirring at this temperature for 3 hours, cool naturally to about 50°C, add dropwise 20ml of toluene solution containing 12.1g of 2,6-dimethylaniline, the reaction is exothermic, control the internal temperature not to exceed 60°C, and react for 2 hours Add 7.3g bromopropane, 4.5gK 2 CO 3 , reacted at 80°C for 4 hours under stirring, filtered hot, and placed the filtrate at 0°C for crystallization. It was filtered and sucked dry, and dried to obtain 12.5 g of white solid.
[0039] Refining: heat-dissolve the crude product with 50ml of toluene, filter, cool, crystallize and filter to obtain 10 g of fine product, with a total yield of 71%.
Embodiment 3
[0041] Mix 6.5g of L-piperidine-2-carboxylic acid with 100ml of toluene, and slowly inject HCl gas under stirring at room temperature until the pH of the reaction solution is 3. When the reaction solution is heated to 55°C, add 0.1ml of DMF, and add 6g of chlorinated chlorinated dropwise at 55°C. Sulfone, after dropping, keep stirring at the temperature for 3 hours, add dropwise 20ml of toluene solution containing 12.1g 2,6-dimethylaniline, the reaction is exothermic, control the internal temperature not to exceed 60°C, add 7.3g bromopropane after 2 hours of reaction , 4.5gK 2 CO 3 , reacted at 70°C for 4 hours under stirring, filtered hot, and placed the filtrate at 0°C for crystallization. It was filtered and sucked dry, and dried to obtain 13.5 g of white solid.
[0042] Refining: heat-dissolve the crude product in 50ml of toluene, filter, cool, crystallize and filter to obtain 11g fine product, with a total yield of 78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com