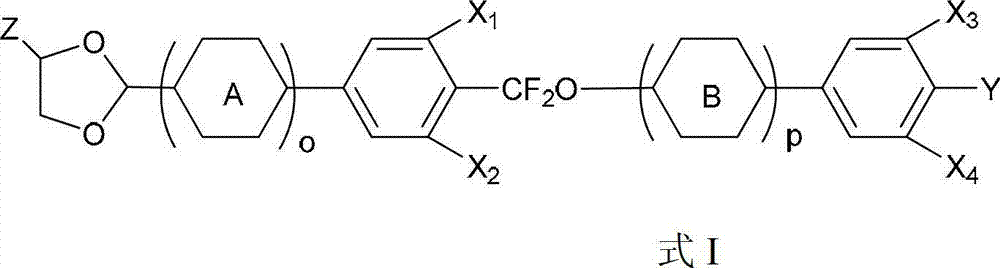
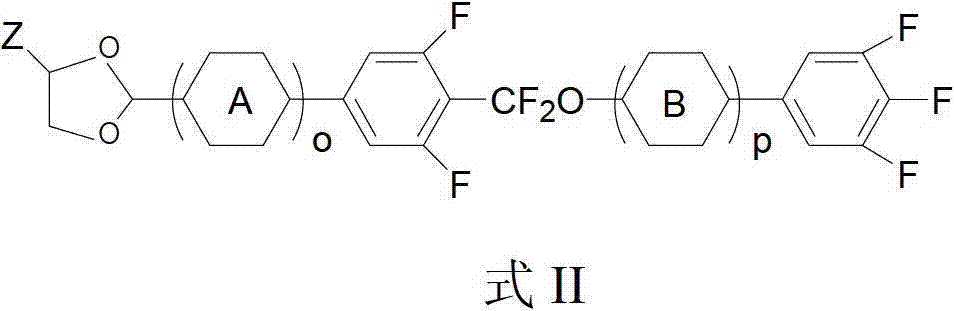
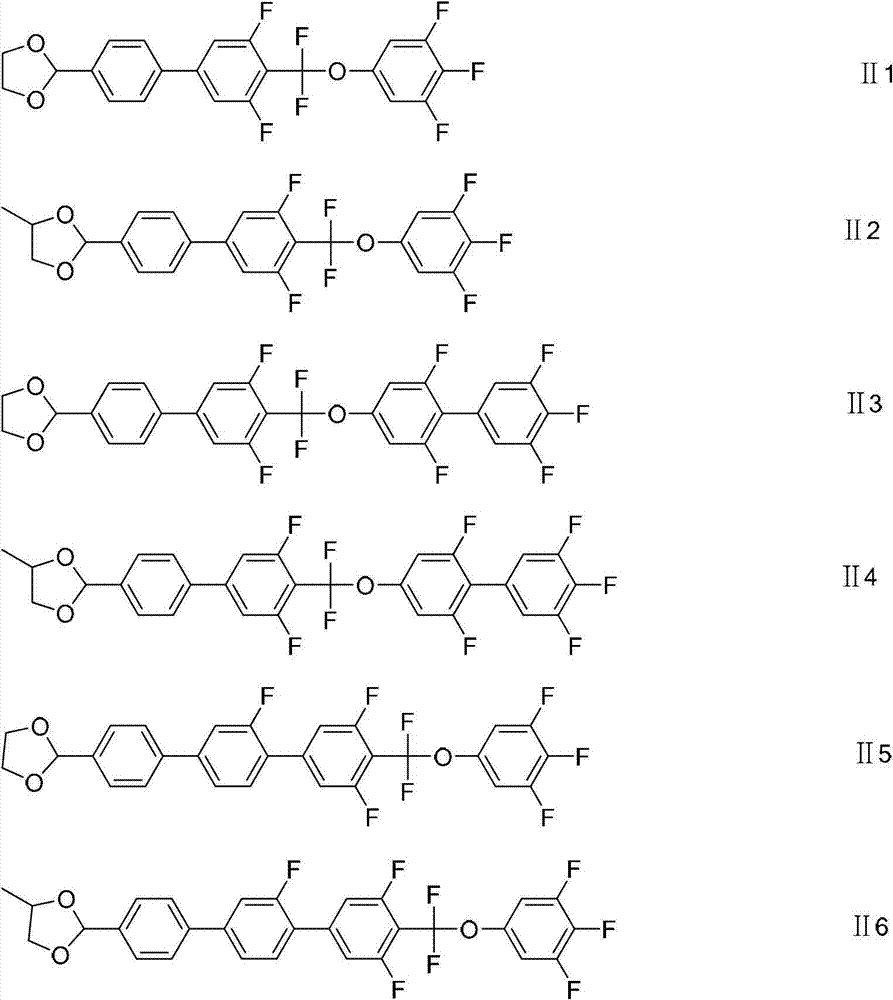
Liquid crystal compound containing 1, 3-dioxolane and difluoro-methylenedioxy linking group and preparation method and application of liquid crystal compound
A compound, phenylene technology, applied in liquid crystal compounds and application fields, can solve the problems of lowering the clearing point of liquid crystal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1, compound 1-d shown in formula I (o is not 0)
[0063]
[0064] step 1
[0065]
[0066] Add 400ml toluene (solvent), 74g (0.4mol) p-bromobenzaldehyde (reactant), 100g (1.6mol) ethylene glycol (reactant), 2.06g (12mmol) p-toluenesulfonic acid (dehydrating agent) to 1L three-necked flask , reflux and divide water for dehydration reaction for 4 hours, no more water will come out, distill the solvent under reduced pressure, dissolve it with petroleum ether, pass through a preheated silica gel column, rinse with petroleum ether, and concentrate the chromatographic solution to obtain a light yellow liquid ( 1-a) 92g, GC: 97%, yield: 98%.
[0067] step 2
[0068]
[0069] Add 92g (0.4mol) (1-a) (reactant), 70g (0.44mol) 3,5-difluorophenylboronic acid (reactant), 300ml toluene (solvent), 300ml ethanol (solvent) to a 2L three-necked flask, 300ml of water (solvent), 53g (0.5mol) of sodium carbonate (alkali), filled with nitrogen to replace the air, added...
Embodiment 2
[0088] Example 2, compound 2-c shown in formula I (o is 0)
[0089]
[0090] step 1
[0091]
[0092] Add 400ml toluene (solvent), 57g (0.4mol) 3,5-difluorobenzaldehyde (reactant), 100g (1.6mol) ethylene glycol (reactant), 2.06g (12mmol) p-toluenesulfonic acid to 1L three-necked flask (dehydrating agent), reflux and divide water for dehydration reaction for 4 hours, no more water will come out, distill the solvent under reduced pressure, dissolve with petroleum ether, pass through the preheated silica gel column, rinse with petroleum ether, and concentrate the chromatographic solution to obtain 75 g of light yellow liquid (2-a), GC: 97%, yield: 98%.
[0093] step 2
[0094]
[0095] Add 75g (0.4mol) (2-a) (reactant) and 500ml tetrahydrofuran (solvent) into a 1L three-neck flask, fill with nitrogen to replace the air, place in a low temperature tank and cool down with liquid nitrogen, when it drops to -60°C, add dropwise 175ml (0.44mol) of 2.5M hexane solution of n-...
Embodiment 3
[0109] With reference to the method of Examples 1 and 2, only the reactant substituents are replaced according to the corresponding substituents in the product to obtain the compound shown in the following formula:
[0110]
[0111] GC: 99.75%
[0112] mp: 43°C
[0113] cp: 21°C
[0114] MS: m / z (%) 472 (M + 4) 325(100) 251(35)
[0115] 1 HNMR (CDCl 3 / TMS):7.383~7.534(dd,4H),7.192~7.280(d,2H),7.062~7.144(d,2H),5.745(s,1H),3.881~4.083(m,3H),1.312~1.323 (d,3H).
[0116] Δn[589nm, 20°C]: 0.147
[0117] Δε[1KHz,20℃]:24.5
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com