Click-chemistry-based pneumococcal polysaccharide conjugate vaccine and preparation method thereof
A technology combining vaccines and pneumonia, applied in the field of biomedicine, can solve the problems of affecting the structural stability of polysaccharides and proteins, affecting the immunogenicity, and long reaction time, so as to reduce the space shielding effect, improve the immunogenicity, and increase the space. effect of distance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
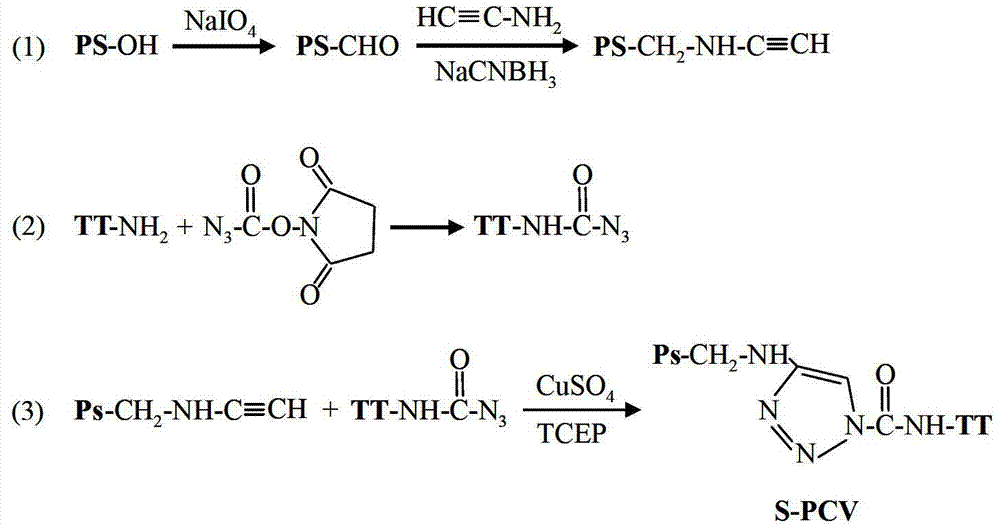
[0031] Example 1: Preparation of short-chain pneumonia capsular polysaccharide conjugate vaccine (S-PCV)
[0032] The preparation reaction of short-chain pneumonia capsular polysaccharide conjugate vaccine (S-PCV) is as follows: figure 1 shown. 5mg of pneumonia capsular polysaccharide was dissolved in 50mM sodium acetate buffer (pH 4.7), and hydrolyzed at 75°C for 30 minutes. The pH value of the hydrolyzate was adjusted to 5.8, and reacted in the presence of sodium periodate at room temperature and protected from light for 10 minutes. Dialysis removes unreacted sodium periodate. The pH of the pretreated pneumonia capsular polysaccharide solution was adjusted to 7.4, and in the presence of sodium cyanoborohydride, the mass ratio of polysaccharide and aminoacetylene was mixed and reacted at a ratio of 2:1, and reacted at 37°C for 6 hours. Centrifuge 3 times at 5000 g using a filter membrane with a molecular weight cut-off of 50 kDa to remove unreacted sodium cyanoborohydride ...
Embodiment 2
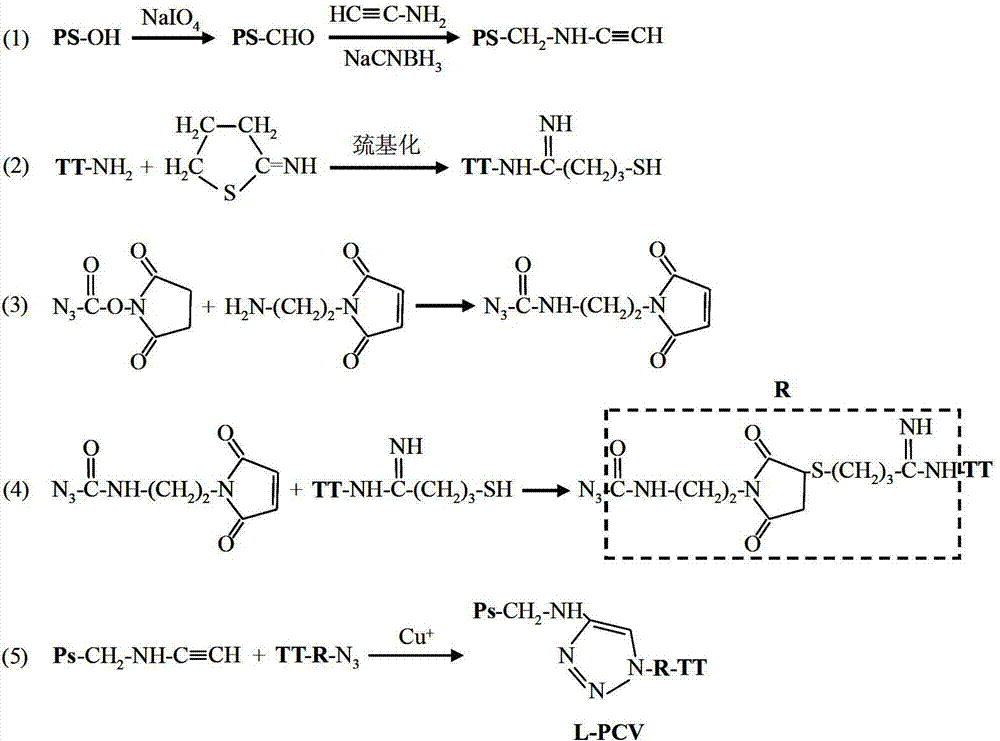
[0034] Example 2: Preparation of long-chain pneumonia capsular polysaccharide conjugate vaccine (L-PCV)
[0035] The preparation reaction of long-chain pneumonia capsular polysaccharide conjugate vaccine (L-PCV) is as follows: figure 2 shown. 5mg of pneumonia capsular polysaccharide was dissolved in 50mM sodium acetate buffer (pH 4.7), and hydrolyzed at 75°C for 30 minutes. The pH value of the hydrolyzate was adjusted to 5.8, and reacted in the presence of sodium periodate at room temperature and protected from light for 10 minutes. Dialysis removes unreacted sodium periodate. The pH of the pretreated pneumonia capsular polysaccharide solution was adjusted to 7.4, and in the presence of sodium cyanoborohydride, the mass ratio of polysaccharide to aminoacetylene was mixed and reacted at a ratio of 2:1, and reacted at 37°C for 6 hours. Centrifuge 3 times at 5000 g using a filter membrane with a molecular weight cut-off of 50 kDa to remove unreacted sodium cyanoborohydride an...
Embodiment 3
[0037] Example 3: Purification and Characterization of L-PCV and S-PCV
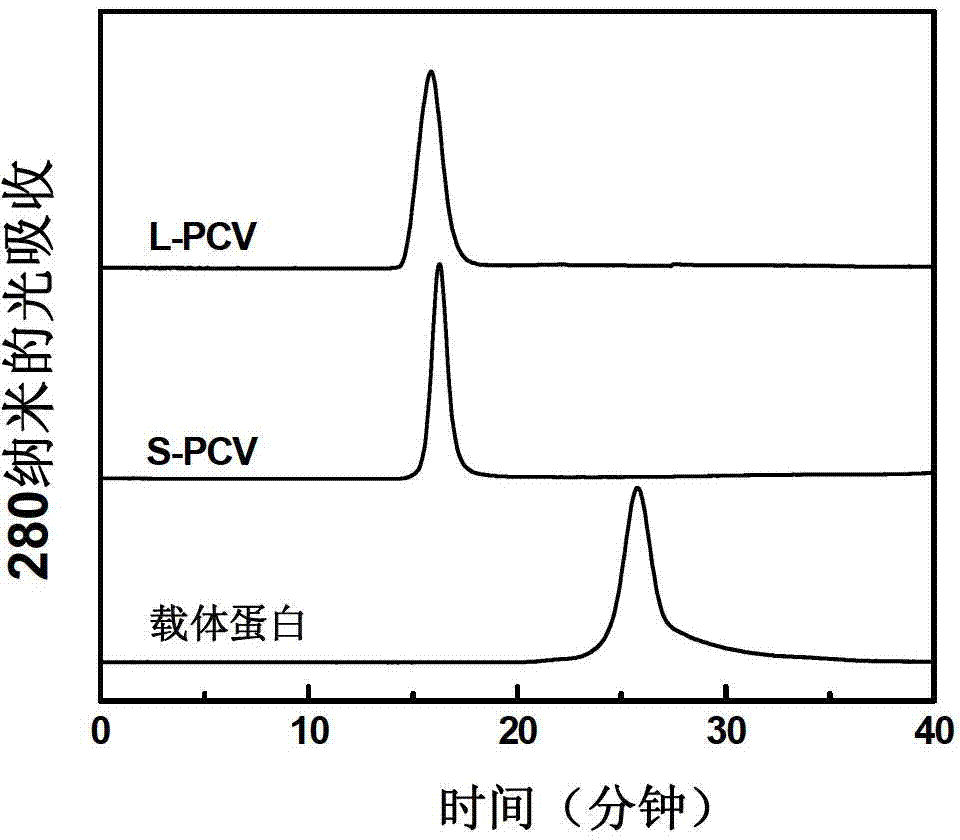
[0038] The binding products involved in Examples 1 and 2 were separated and purified with a Superdex200 gel filtration column (2.6cm×60cm), the eluent was 20mM phosphate buffer (pH7.4), and the flow rate was 3ml / min . Elution peaks corresponding to L-PCV and S-PCV were collected separately.
[0039] The purified product was identified by Superdex200 gel filtration column (1.0cm×30cm), the eluent was 20mM phosphate buffer (pH7.4), and the flow rate was 0.5ml / min. Such as image 3 As shown, compared with the carrier protein, the peak time of L-PCV and S-PCV was significantly earlier. This indicates that the molecular weight of the carrier protein increases significantly after binding to the pneumonia capsular polysaccharide.
[0040] use 1 H-NMR was performed to detect L-PCV and S-PCV. Such as Figure 4 As shown, compared with pneumonia polysaccharide molecules, S-PCV has protein characteristic peaks...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com