2-aryl quinazoline or 2-heterocyclic aryl quinazoline derivative and preparation method thereof
A technology of heterocyclic aryl quinazoline and aryl quinazoline is applied in the field of preparation of 2-aryl quinazoline or 2-heterocyclic aryl quinazoline derivatives, and can solve the problem of high corrosiveness of equipment, It is difficult to separate and purify, and the reaction conditions are demanding, so as to achieve the effects of easy purification, high yield and simple synthesis method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The preparation method of above-mentioned 2-aryl quinazoline or 2-heterocyclic aryl quinazoline derivative is characterized in that comprising the following steps:
[0030]
[0031] a. In the presence of a catalyst, aromatic formaldehyde or heterocyclic aromatic formaldehyde reacts with anthranilamide to obtain a class of 2-arylquinazolin-4(3H)-one derivatives or 2 as shown in formula (2) -Heterocyclic arylquinazolin-4(3H)-one derivatives;
[0032] b. 2-arylquinazolin-4(3H)-one derivatives or 2-heterocyclic arylquinazolin-4(3H)-one derivatives are reduced by a reducing agent to obtain a class such as formula (3) Represented 2-aryl-3, 4-dihydroquinazoline derivatives or 2-heterocyclic aryl-3, 4-dihydroquinazoline derivatives;
[0033] c. Under oxidizing conditions, 2-aryl-3, 4-dihydroquinazoline derivatives or 2-heterocyclic aryl-3, 4-dihydroquinazoline derivatives are dehydrogenated to obtain a class such as (i) represented 2-arylquinazoline or 2-heterocyclic arylq...
Embodiment 1
[0038] Example 1: Preparation of 2-(4-(diphenylamino)phenyl)quinazolin-4(3H)-one
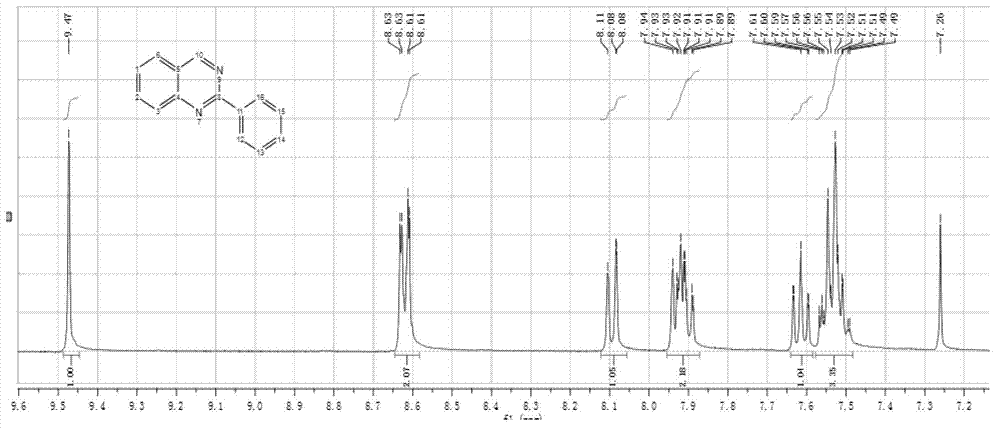
[0039] Weigh 4.08 g (30 mmol) anthranilamide and 6.24 g (60 mmol) NaHSO 3 Add two-neck bottle, N 2 Add 30 mL of N,N-dimethylacetamide and 8.18 g (30 mmol) of 4-(diphenylamino)benzaldehyde under protection, raise the temperature to 150°C and stir for 8 h. After the reaction is complete, cool to room temperature. The reaction solution was poured into a large amount of water, a white precipitate appeared, suction filtered, washed with water and ethanol respectively, and dried to obtain 2-(4-(diphenylamino)phenyl)quinazolin-4(3H)-one in the form of white crystals . Yiled: 95.7%. 1 H NMR (400 MHz, DMSO- d 6 )δ(ppm): 8.07-8.11 (m, 3H), 7.76-7.80 (m, 1H), 7.65-7.67 (d, 1H), 7.43-7.47 (m, 1H), 7.35-7.39 (m, 4H) , 7.11-7.17 (m, 6H), 6.94-6.96 (d, 2H).
Embodiment 2
[0040] Embodiment 2: the preparation of compound N,N-diphenyl-4-(quinazolin-2-yl)aniline (TPAQx)
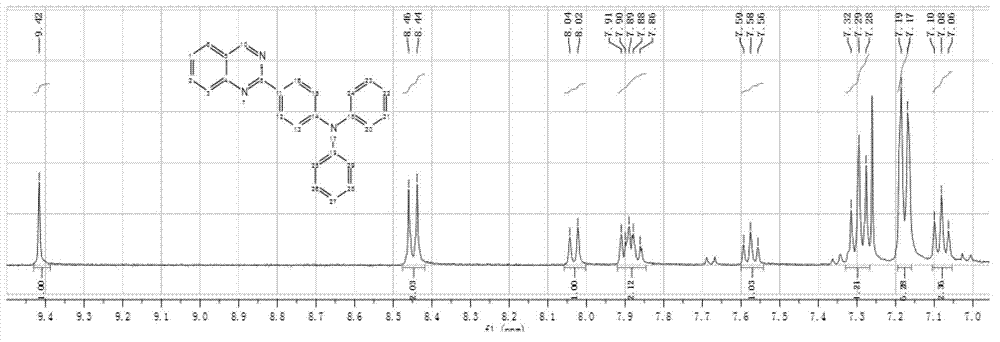
[0041]Weigh 0.39 g (10.24 mmol) LiAlH 4 Add it to a two-neck flask, add 5 mL tetrahydrofuran (THF) under nitrogen protection, and dissolve 1 g (2.56 mmol) 2-(4-(diphenylamino)phenyl)quinazolin-4(3H)-one in THF Slowly add it dropwise into the reaction flask, raise the temperature to 70°C and stir for 12 hours, after the reaction is completed, cool to room temperature, pour the reaction solution into a large amount of water, extract with ethyl acetate, and purify by silica gel column chromatography to obtain 2-(4- (diphenylamino)phenyl)-3,4-dihydroquinazoline. Yield: 72.5%. 1 H NMR (400 MHz, DMSO- d 6 )δ(ppm): 8.07-8.11 (m, 3H), 7.76-7.80 (m, 1H), 7.65-7.67 (d, 1H), 7.43-7.47 (m, 1H), 7.35-7.39 (m, 4H) , 7.11-7.17 (m, 6H), 6.94-6.96 (d, 2H).
[0042] Weigh 0.3 g (0.8 mmol) of 2-(4-(diphenylamino)phenyl)-3,4-dihydroquinazoline and 0.14 g (1.6 mmol) of manganese dioxide into a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com