Preparation method for cobalt boroacylate with low melting point
A technology of cobalt boroacylate and low melting point, which is applied in the field of preparation of cobalt boroacylate with low melting point to achieve the effect of good dispersion and low melting point
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
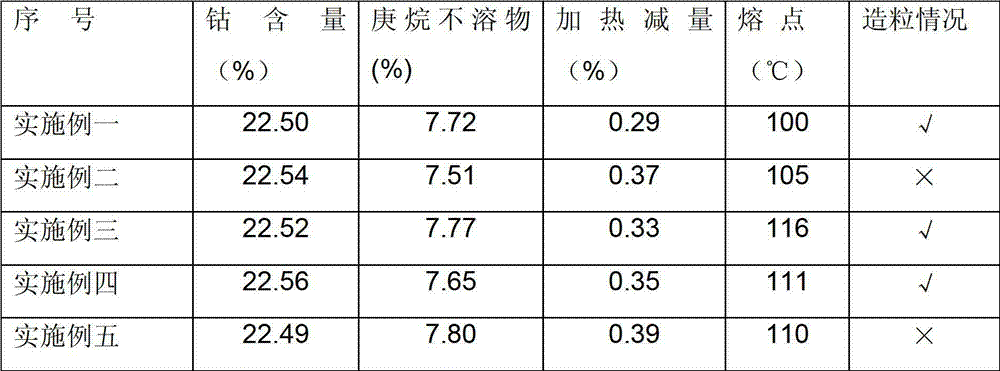
Examples
Embodiment 1
[0043] This embodiment provides a method for preparing cobalt boroacylate, which can be used as an accelerator for rubber composite materials with excellent performance. The method includes:
[0044] Mix neodecanoic acid, propionic acid, valeric acid, stearic acid 1845, rosin, xylene and cobalt hydroxide to carry out acid-base neutralization reaction, and obtain an intermediate product after the neutralization reaction is completed;
[0045] Add tributyl borate to the obtained intermediate product to carry out boroacylation reaction, and at the same time add calcium borate as an additive, and the reaction product obtained after the boroacylation reaction is completed is cobalt boroacylate.
[0046] Above-mentioned method specifically comprises two steps of neutralization reaction and boroacylation reaction:
[0047] Neutralization reaction: Stir and mix neodecanoic acid, valeric acid, stearic acid 1845, rosin, and xylene, then add cobalt hydroxide while stirring and vacuuming;...
Embodiment 2
[0064] This embodiment provides a method for preparing cobalt boroacylate, and the prepared cobalt boroacylate can be used as an accelerator for rubber composite materials. The method includes:
[0065] Neutralization reaction: 40 to 45 parts by weight of neodecanoic acid, 1 to 4 parts by weight of valeric acid, 6 to 8 parts by weight of stearic acid 1845, 1 to 4 parts by weight of rosin and 40 to 50 parts by weight of xylene Add it into the reaction kettle, start high-speed stirring, and its rotating speed is 150-250 rpm, and at the same time slowly add 35-38 parts by weight of cobalt hydroxide, after adding cobalt hydroxide, slowly raise the temperature and dropwise add 28 parts by weight of acrylic acid acid; when the temperature rises to 93°C, xylene and water are distilled out; when the temperature rises to 160°C, vacuum distillation is carried out, and the vacuum is reduced from -0.01MPa to -0.08MPa during the decompression process, and the vacuum is -0.08MPa After keepi...
Embodiment 3
[0069] This embodiment provides a method for preparing cobalt boroacylate, and the prepared cobalt boroacylate can be used as an accelerator for rubber composite materials. The method includes:
[0070] Neutralization reaction: adding 40-45 parts by weight of neodecanoic acid, 0 parts by weight of valeric acid, 6-8 parts by weight of stearic acid 1845, 1-4 parts by weight of rosin and 40-50 parts by weight of xylene In the reaction kettle, start high-speed stirring at a speed of 150 to 250 rpm, and at the same time slowly add 35 to 38 parts by weight of cobalt hydroxide. After adding cobalt hydroxide, slowly heat up and dropwise add 30 to 35 parts by weight of acid; when the temperature rises to 93°C, xylene and water are distilled out; when the temperature rises to 160°C, vacuum distillation is carried out, and the vacuum is reduced from -0.01MPa to -0.08MPa during the decompression process, and the vacuum is -0.08MPa After keeping for 120 minutes, the acid-base neutralizatio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com