Polyglutamic acid derivative as well as hydrogel and preparation method of polyglutamic acid derivative
A technology of polyglutamic acid and derivatives, which is applied in the fields of pharmaceutical formulations, medical preparations of non-active ingredients, and pharmaceutical sciences. It can solve the limitations of the application field, which has not been reported before, and the mechanical strength of the copolypeptide gel is low. and other problems, to achieve the effect of mature synthesis method, simple process and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
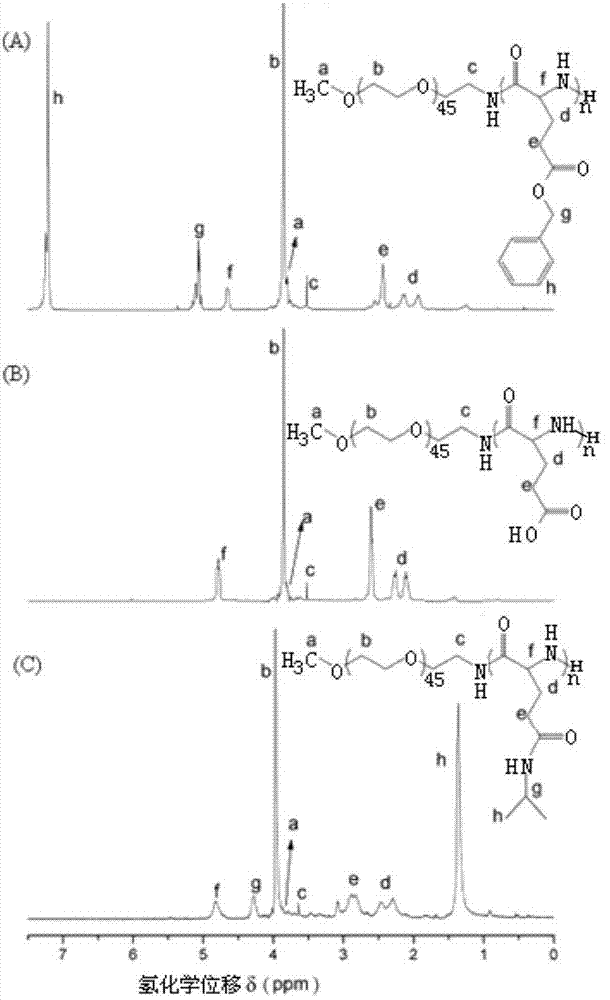
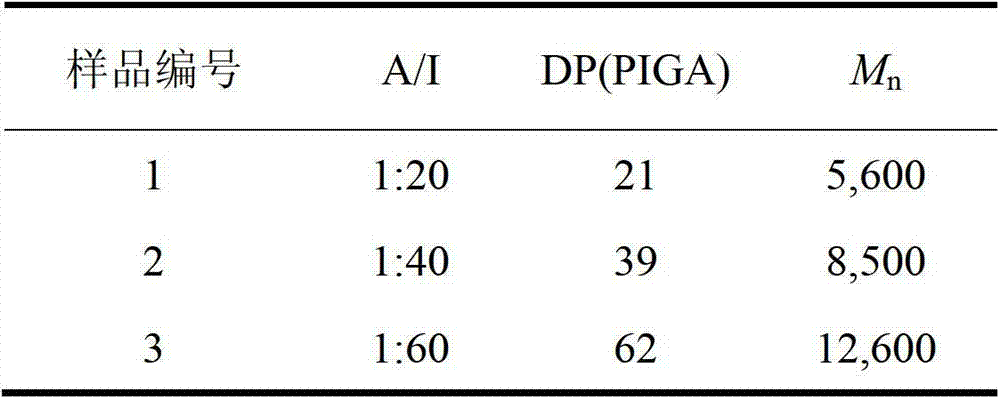
[0031] The polyglutamic acid derivative of this embodiment is polyethylene glycol monomethyl ether-b-polyglutamic acid derivative modified by isopropylamine, specifically polyethylene glycol monomethyl ether-b-poly(N-isopropyl base-L-glutamine), which was prepared as follows:
[0032] (1) Preparation of polyethylene glycol monomethyl ether-b-polybenzyl L-glutamate (mPEG-b-PBLG) block copolymer
[0033] Under the protection of nitrogen, the amino-terminated polyethylene glycol monomethyl ether was dissolved in anhydrous N,N-dimethylformamide to initiate L-benzyl glutamate-N-carboxylate anhydride (BLG-NCA ) in a nitrogen environment at room temperature (15-25 ° C) closed ring-opening polymerization for 72 hours, the reaction solution was settled in excess anhydrous ether, and a white solid was obtained by suction filtration, and then vacuum-dried to constant weight; polyethylene glycol monomethyl Ether-b-polybenzyl glutamate block copolymer.
[0034] Among them, benzyl L-gluta...
Embodiment 2
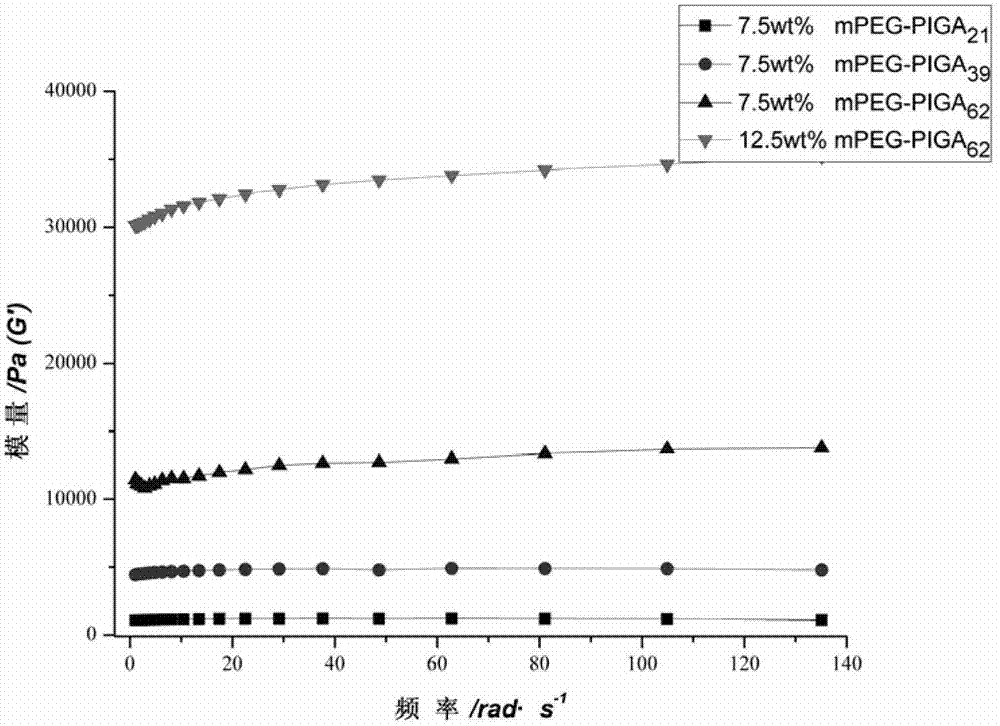
[0045] Preparation of polyethylene glycol monomethyl ether-b-poly(N-isopropyl-L-glutamine) hydrogel:
[0046] (1) Configuration of pH=7.4 phosphate buffer solution
[0047] Accurately weigh 9.078g of potassium dihydrogen phosphate and 23.876g of disodium hydrogen phosphate, dissolve them in 1000mL of double distilled water and stir evenly, then take 800mL of disodium hydrogen phosphate solution and 200mL of potassium dihydrogen phosphate solution in a 1000mL volumetric flask Constant volume was obtained to obtain a phosphate buffer solution with pH=7.4.
[0048] (2) Preparation of polyethylene glycol monomethyl ether-b-poly(N-isopropyl-L-glutamine) hydrogel
[0049] Dissolve polyethylene glycol monomethyl ether-b-poly(N-isopropyl-L-glutamine) in phosphate buffer solution with pH=7.4 to make a viscous solution and put it in a constant temperature shaking box at 37°C After 2 hours, the hydrogel of the polymer was formed; the formation of the hydrogel was macroscopically charac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com