Isosorbide mononitrate derivative and preparation method and application thereof
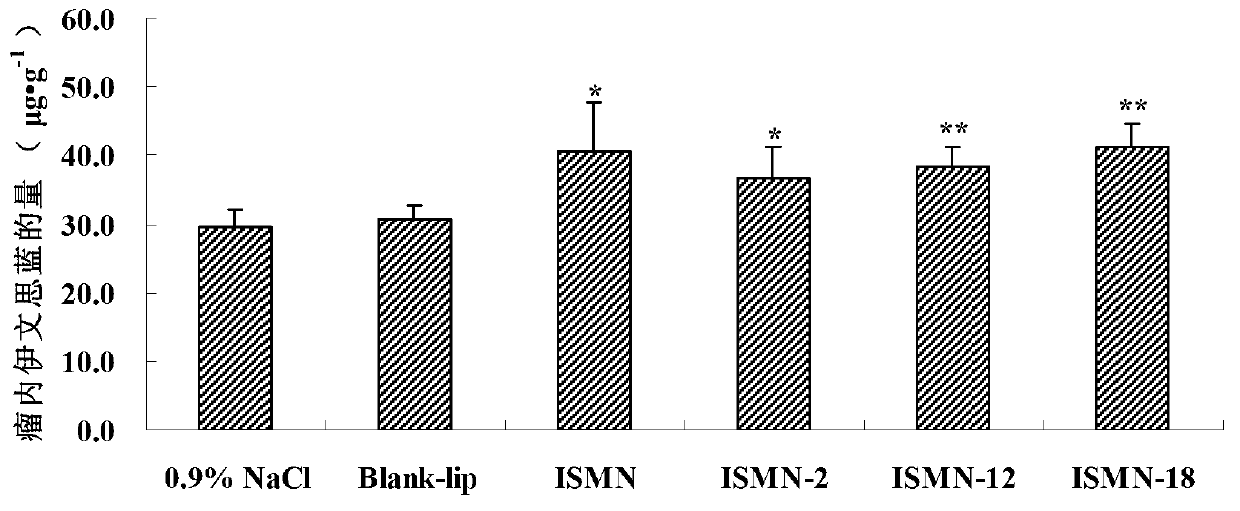
A technology of isosorbide dinitrate and its derivatives, which is applied in the field of isosorbide mononitrate derivatives and its preparation and application, can solve the problems of high water solubility and poor skin permeability of isosorbide mononitrate, and achieve enhanced anticancer efficacy , The effect of improving fat solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
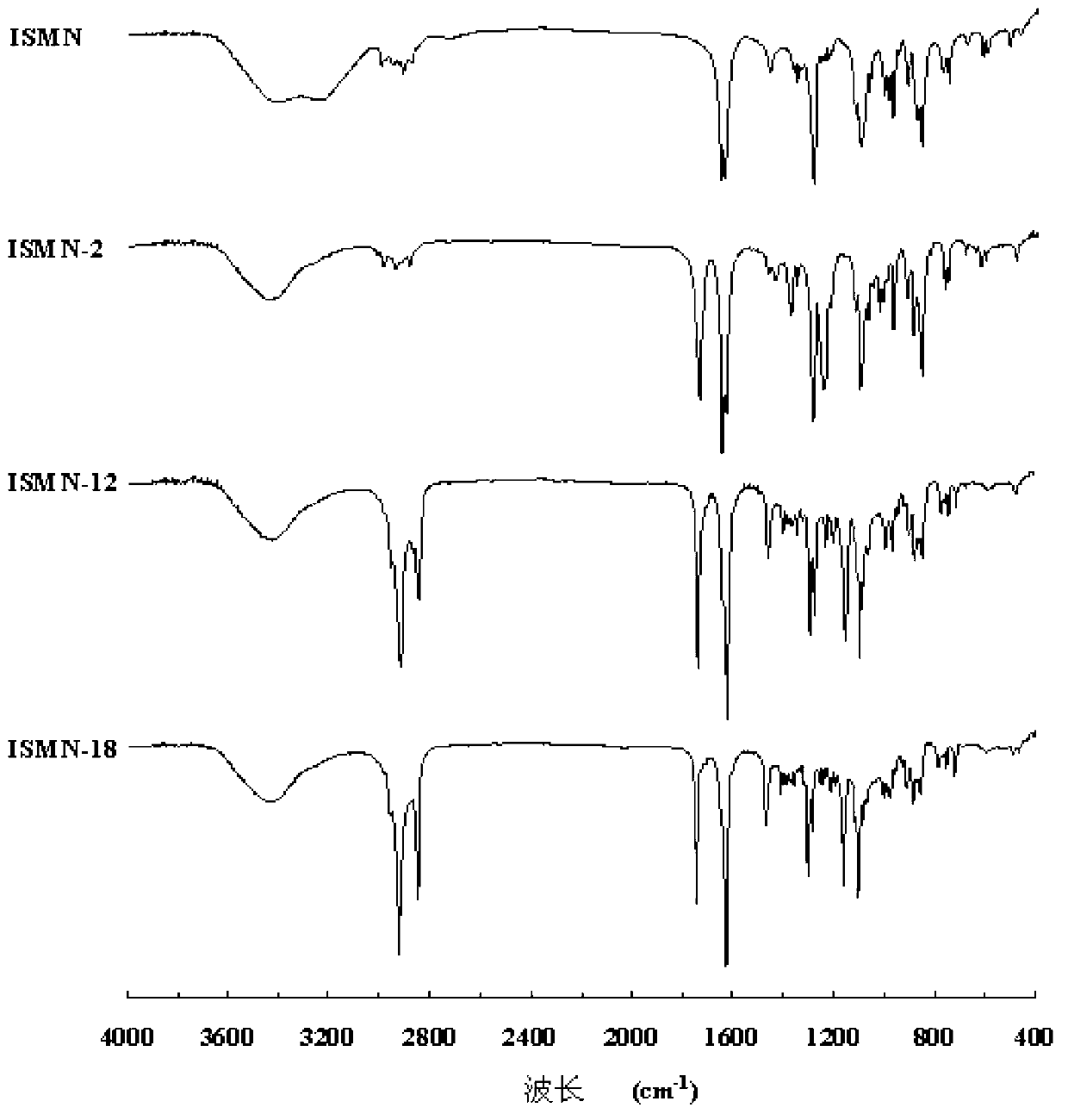
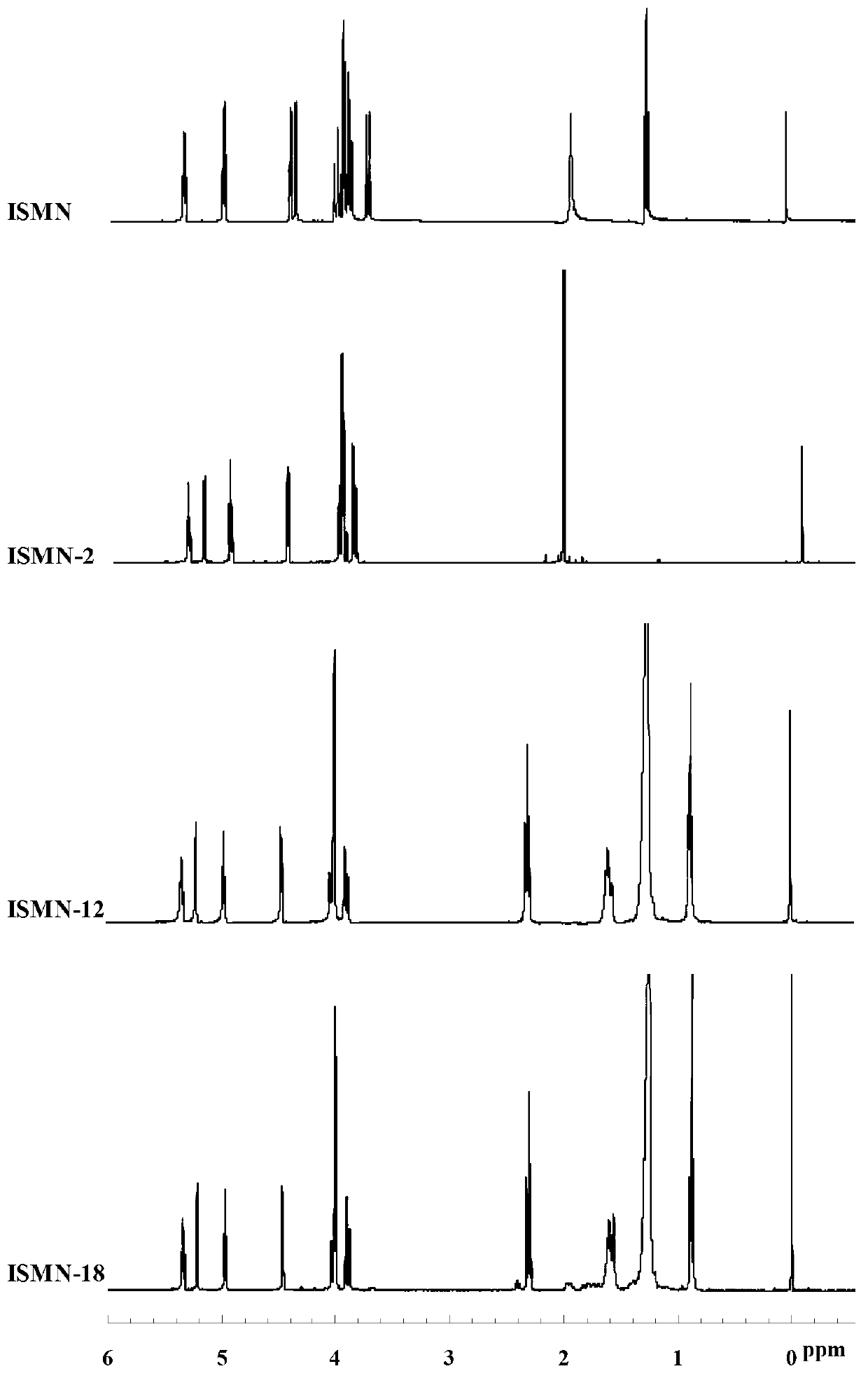
[0038] Embodiment 1 Preparation of isosorbide mononitrate acetic acid derivative (ISMN-2)
[0039] Weigh 955mg (5mmol) of isosorbide mononitrate (ISMN for short), put it in a 100mL round bottom flask, add 50ml of anhydrous dichloromethane to dissolve; then add 612mg (6mmol) of acetic anhydride and 67mg of 4-N , N-dimethylaminopyridine (DMAP), magnetically stirred, and reacted at room temperature for 15 hours. The reaction solution was washed 3 times with 250mL saturated NaHCO3 solution, 3 times with 250mL deionized water, the organic layer was collected, and then the moisture in the organic layer was removed with anhydrous Na2CO3, Na2CO3 was removed by filtration, and the organic solvent was removed by rotary evaporation of the filtrate under reduced pressure to obtain the product 779mg, yield 49.7%, recorded as ISMN-2.
Embodiment 2
[0040] Example 2 Preparation of Isosorbide Mononitrate Lauric Acid Derivatives (ISMN-12)
[0041] Weigh 955mg (5mmol) of isosorbide mononitrate (ISMN), put it in a 100mL round bottom flask, add 50ml of anhydrous dichloromethane to dissolve; then add 1.2g (6mmol) of lauric acid, 67mg of 4-N , N-dimethylaminopyridine (DMAP) and 1.1g of dehydrating agent 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDCI), magnetic stirring, room temperature for 10 hours . The reaction solution was washed with 250mL saturated NaHCO 3 Solution was washed 3 times, 250mL deionized water was washed 3 times, the organic layer was collected, and then washed with anhydrous Na 2 CO 3 Remove the moisture in the organic layer, filter to remove Na 2 CO 3 , The organic solvent was removed by rotary evaporation of the filtrate under reduced pressure to obtain 1.6 g of the product with a yield of 76.5%, which was designated as ISMN-12.
Embodiment 3
[0042] Example 3 Preparation of isosorbide mononitrate stearic acid derivative (ISMN-18)
[0043] Weigh 955mg (5mmol) of isosorbide 5-mononitrate (ISMN), put it in a 100mL round bottom flask, add 50ml of anhydrous dichloromethane to dissolve; then add 1.7g (6mmol) of stearic acid, 67mg of 4-N,N-dimethylaminopyridine (DMAP) and 1.1 g of dehydrating agent 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDCI), magnetic stirring, room temperature React for 12 hours. The reaction solution was washed with 250mL saturated NaHCO 3 Solution was washed 3 times, 250mL deionized water was washed 3 times, the organic layer was collected, and then washed with anhydrous Na 2 CO 3 Remove the moisture in the organic layer, filter to remove Na 2 CO 3 , The organic solvent was removed by rotary evaporation of the filtrate under reduced pressure to obtain 1.7 g of the product with a yield of 64.6%, which was designated as ISMN-18.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com