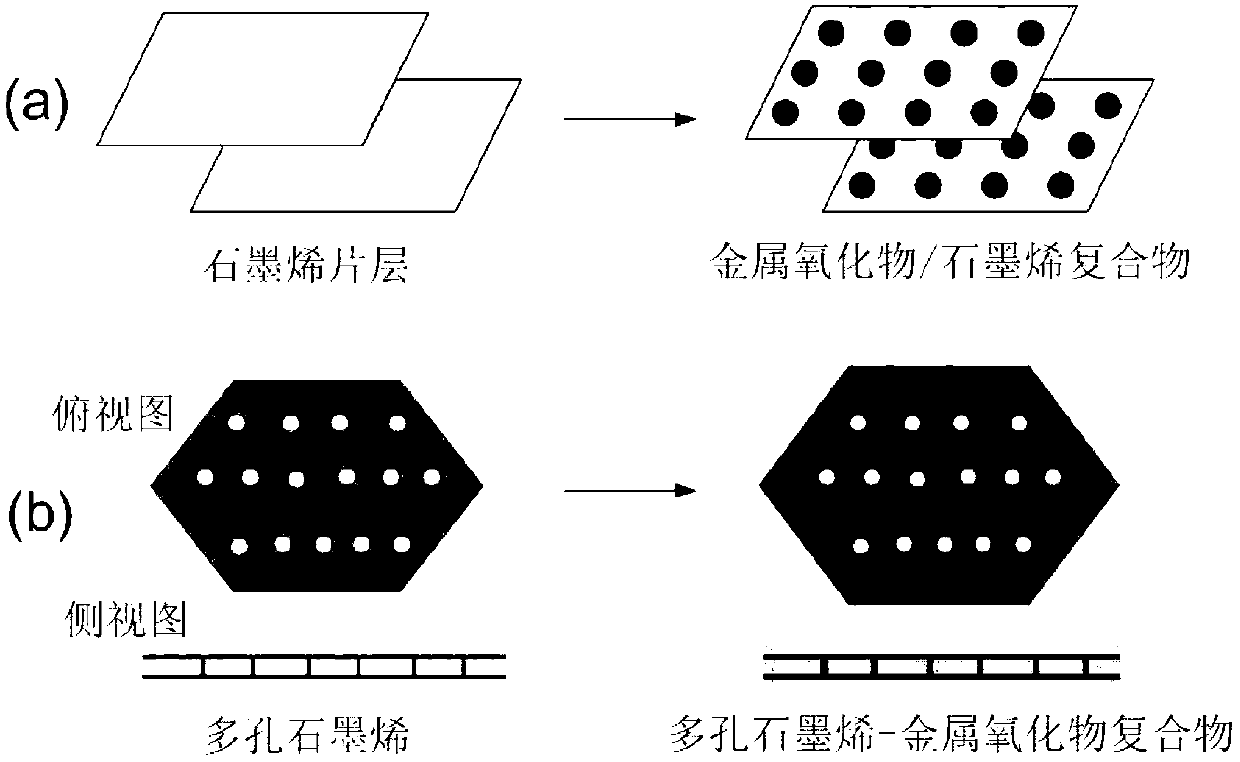
Porous graphene-metal oxide composite material and its preparation method
A technology of porous graphene and composite materials, applied in electrical components, battery electrodes, circuits, etc., to achieve high specific capacity, prevent coalescence, and improve stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Using ion exchange and liquid deposition processes to convert Co 3 O 4 Loaded into the mesopores of porous graphene to obtain porous graphene and Co 3 O 4 Composite material, where Co 3 O 4 Accounting for 70% (wt%) of the total weight of the composite material, the composite material is expressed as 70% Co-porous graphene.
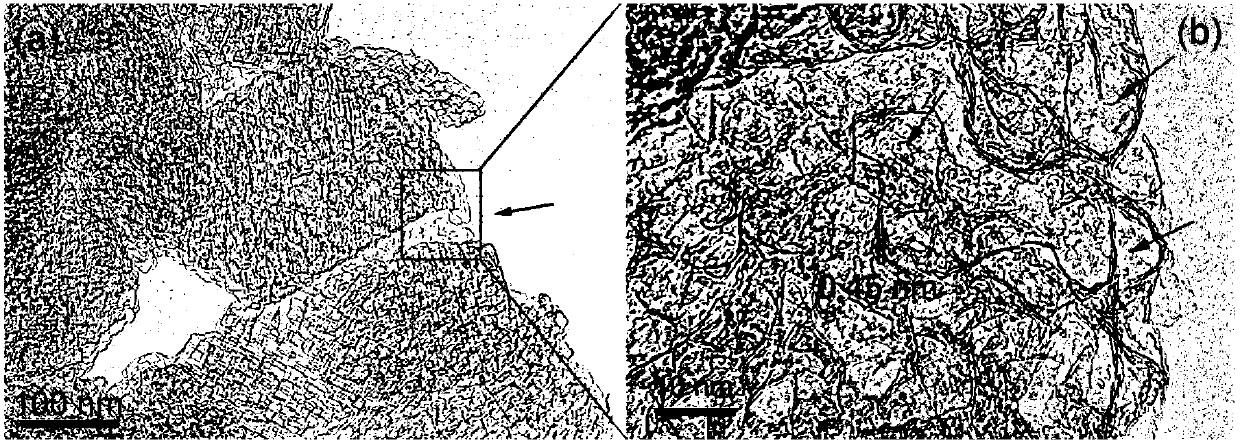
[0049] The porous graphene used in this example was prepared by the method described in Example 1 of CN 102115069 A. The TEM picture of the sample is as follows figure 1 Shown.
[0050] Use the method described in CN 102115069 A to prepare porous flake MgO, take 100g of porous flake MgO into a fixed bed reactor, heat it to 850°C in an Ar atmosphere, then pass in methane and react for 10 minutes. Cooled to room temperature in an argon atmosphere, the gray black powder obtained is the graphene-MgO composite obtained by the vapor chemical deposition method.
[0051] Preparation of porous graphene and Co 3 O 4 The composite material process includes two main s...
Embodiment 2
[0061] Using ion exchange and liquid deposition 2 O 3 Loaded into the mesopores of porous graphene to obtain porous graphene and Fe 2 O 3 Composite material, where Fe 2 O 3 10% (wt%) of the total weight of the composite material, the composite material is expressed as 10% Fe 2 O 3 -Porous graphene.
[0062] The same process as in Example 1 was used, except that the template agent was replaced with MgSO 4 Whiskers (prepared according to the method in the literature Crystal Research and Technology 2008; 43(5): 479-482) to prepare graphene-MgSO 4 Complex. 30g graphene-MgSO 4 Disperse the complex in excess dilute hydrochloric acid (concentrated hydrochloric acid: water = 1:3, volume ratio), stir evenly, transfer the suspension to a round bottom flask, install a condenser, boil and reflux for 1 hour and then cool to room temperature; Then vacuum filtration, disperse the obtained filter cake into deionized water, and hydrothermally treat it at 50°C for 30 minutes to remove MgCl in the p...
Embodiment 3
[0069] In this embodiment, the Co 3 O 4 Loaded into the mesopores of porous graphene (porous graphene) to obtain porous graphene and Co 3 O 4 Composite material, where Co 3 O 4 Accounting for 76% of the total weight of the composite material, the composite material is expressed as 76% Co-porous graphene.
[0070] First, the same process as in Example 1 was used to prepare the graphene-MgO composite. Disperse 30 g of graphene-MgO composite obtained by vapor chemical deposition in 800 mL of dilute hydrochloric acid (concentrated hydrochloric acid: water = 1:3, volume ratio), stir evenly, transfer the suspension to a round bottom flask, and install it Condensate tube, boil and reflux for 1 hour and then cool to room temperature; then vacuum filter, disperse the resulting filter cake in deionized water, and hydrothermally heat it at 150 degrees Celsius for 2 hours; vacuum filter again to obtain a porous graphene filter cake with water.
[0071] Add 26g Co(NO 3 ) 2 ·6H 2 O was dissolved...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com