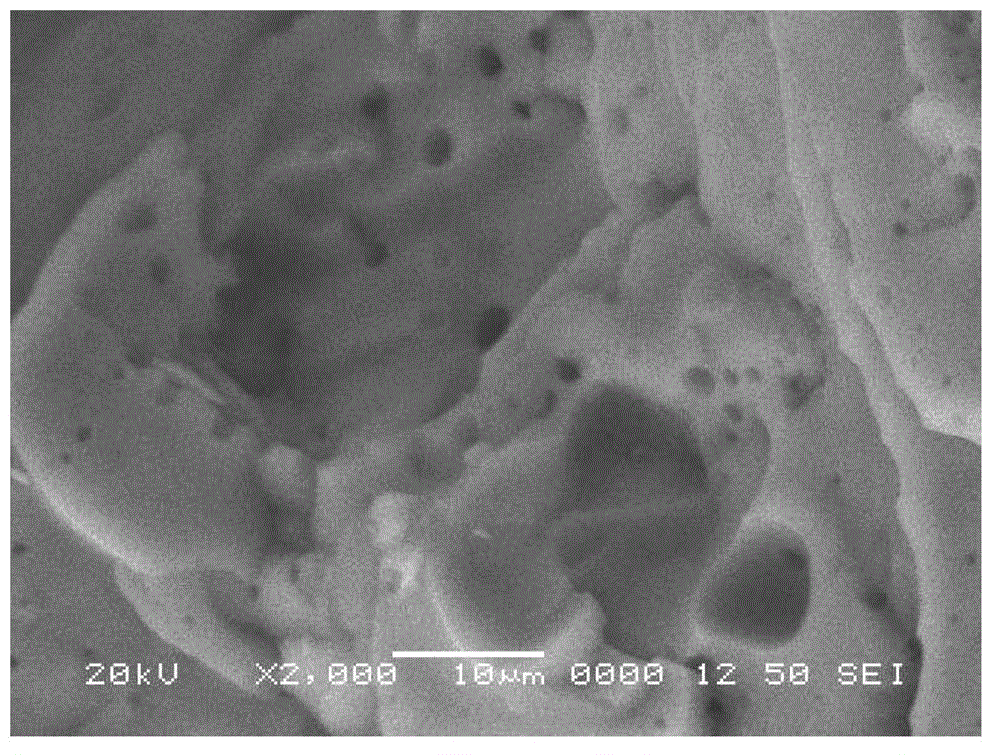
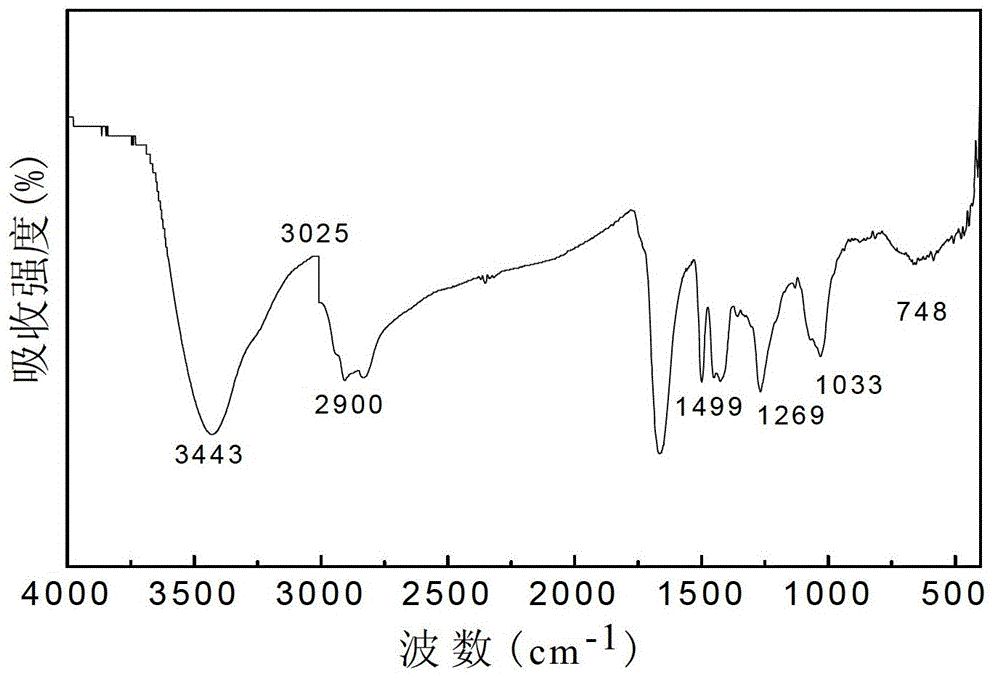
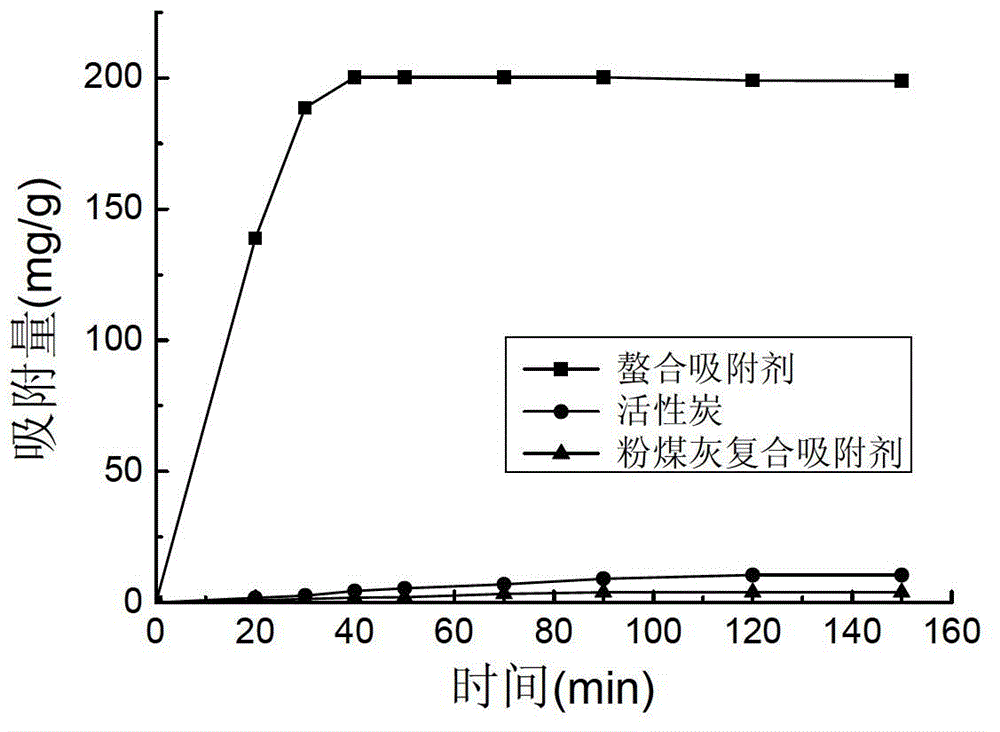
Preparation method of heavy metal chelating adsorbent
A technology for chelating adsorbents and heavy metals, which is applied in the field of preparation of heavy metal chelating adsorbents, can solve the problems of limited use range, low adsorption regeneration rate, high operating cost, etc. Simple method, good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Taking raw material diethylenetriamine 11mL as example, the method for preparing this heavy metal chelating adsorbent consists of the following steps:
[0023] (1) Take 10g of sodium hydroxide to prepare a solution with a concentration of 2.5mol / L, stir and wait for cooling, then add 11mL of diethylenetriamine and 14mL of carbon disulfide, the molar ratio of diethylenetriamine to carbon disulfide and sodium hydroxide is 1 :2.3:2.5, stir and mix well, react at room temperature for 1h, until the reaction solution is orange-yellow.
[0024] (2) Add 10mL of epichlorohydrin dropwise to the reaction solution at a rate of 1 drop / s, the molar ratio of diethylenetriamine to epichlorohydrin is 1:1, stir and mix well, and heat at 60°C In a water bath, react for 4 hours to obtain a solid product.
[0025] (3) Take out the solid product and wash it once with distilled water, once with acetone, twice with ethanol, and then with distilled water until the pH of the product is 7.0 to p...
Embodiment 2
[0027] Taking 11mL of raw material diethylenetriamine as an example, the method for preparing heavy metal chelating adsorbent consists of the following steps:
[0028] (1) Take 10g of sodium hydroxide to prepare a solution with a concentration of 2.5mol / L, stir and wait for cooling, then add 11mL of diethylenetriamine and 12mL of carbon disulfide, the molar ratio of diethylenetriamine to carbon disulfide and sodium hydroxide is 1 : 1.99:2.5, stirred and mixed, reacted at room temperature for 1h, until the reaction solution was orange-yellow.
[0029] (2) Add 8mL of epichlorohydrin dropwise to the reaction solution at a rate of 1 drop / s, the molar ratio of diethylenetriamine to epichlorohydrin is 1:0.8, stir and mix well, and heat at 60°C In a water bath, react for 4 hours to obtain a solid product.
[0030] Other steps are the same as in Example 1 to prepare a heavy metal chelating adsorbent.
Embodiment 3
[0032] Taking 11mL of raw material diethylenetriamine as an example, the method for preparing heavy metal chelating adsorbent consists of the following steps:
[0033] (1) Take 10g of sodium hydroxide to prepare a solution with a concentration of 2.5mol / L, stir and wait for cooling, then add 11mL of diethylenetriamine and 16mL of carbon disulfide, the molar ratio of diethylenetriamine to carbon disulfide and sodium hydroxide is 1 :2.65:2.5, stirred and mixed, reacted at room temperature for 1h, until the reaction solution was orange-yellow.
[0034] (2) Add 12mL of epichlorohydrin dropwise to the reaction solution at a rate of 1 drop / s, the molar ratio of diethylenetriamine to epichlorohydrin is 1:1.2, stir and mix well, and heat at 60°C In a water bath, react for 4 hours to obtain a solid product.
[0035] Other steps are the same as in Example 1 to prepare a heavy metal chelating adsorbent.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com