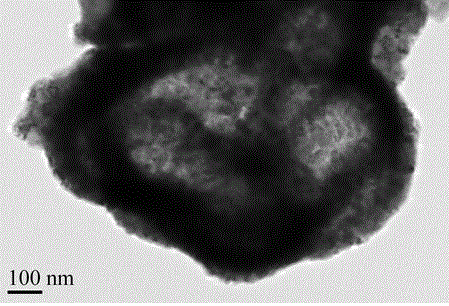
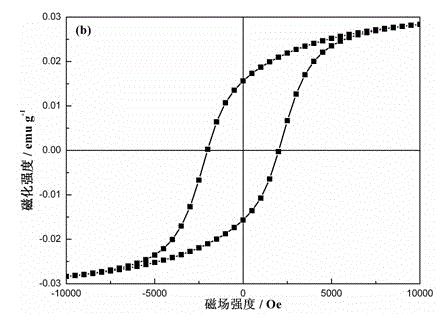
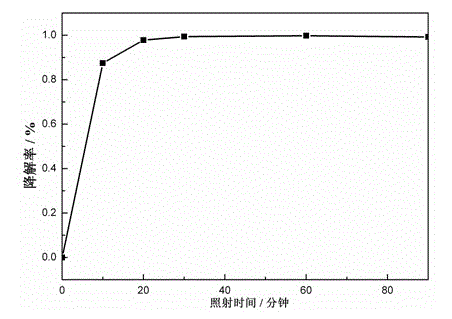
Magnetic recyclable hollow TiO2-SiO2-CoFe2O4 nano photocatalytic material and preparation method thereof
A tio2-sio2-cofe2o4, catalytic material technology, applied in the field of nano-photocatalytic materials, can solve the problems of reduced magnetic properties of photocatalysts, reduced activity of photocatalysts, not seen, etc., achieves low density, strengthens the effect of TiO2 bonding, Not easy to fall off effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0037] 1) The 0.05 g / mL glucose solution was hydrothermally synthesized at a hydrothermal temperature of 200 °C for 4 h to prepare carbon spheres;
[0038] 2) Prepare a 0.003 g / mL NaCl solution, add polymethyldiallyl ammonium chloride, namely PDDA, to prepare a 0.1 wt.% PDDA solution, add the product obtained in the above step 1) and stir for 60 min before filtering, Obtain carbon sphere-PDDA; prepare 0.003 g / mL NaCl solution, add polystyrene sulfonate sodium, namely PSS, to prepare 0.05 wt.% PSS solution, add carbon sphere-PDDA and stir for 30 min, then filter to obtain carbon Balls-(PDDA-PSS); adding carbon balls-(PDDA-PSS) to the above PDDA solution to prepare carbon balls-(PDDA-PSS-PDDA) particles, finally making the surface of carbon balls uniformly distributed positive charges;
[0039] 3) Add 0.045 g / mL FeCl 3 ·6H 2 O and 0.02 g / mL CoCl 2 ·6H 2 O dissolved in ethylene glycol, where FeCl 3 ·6H 2 O and CoCl 2 ·6H 2 The molar ratio of O is 2:1; then urea and polyvi...
Embodiment 3
[0045] 1) The 0.5 g / mL glucose solution was hydrothermally synthesized at a hydrothermal temperature of 150 °C for 12 h to prepare carbon spheres;
[0046] 2) Prepare a 0.005 g / mL NaCl solution, add polymethyldiallyl ammonium chloride, namely PDDA, to prepare a 0.2 wt.% PDDA solution, add the product obtained in the above step 1) and stir for 60 min before filtering, Obtain carbon spheres-PDDA; prepare 0.005 g / mL NaCl solution, add polystyrene sulfonate sodium, namely PSS, to prepare a 0.1 wt.% PSS solution, add carbon spheres-PDDA and stir for 30 min, then filter to obtain carbon Balls-(PDDA-PSS); adding carbon balls-(PDDA-PSS) to the above PDDA solution to prepare carbon balls-(PDDA-PSS-PDDA) particles, finally making the surface of carbon balls uniformly distributed positive charges;
[0047] 3) Add 0.405 g / mL FeCl 3 ·6H 2 O and 0.18 g / mL CoCl 2 ·6H 2 O dissolved in ethylene glycol, where FeCl 3 ·6H 2 O and CoCl 2 ·6H 2 The molar ratio of O is 2:1; then urea and pol...
Embodiment 4
[0053] 1) The 0.45 g / mL glucose solution was hydrothermally synthesized at a hydrothermal temperature of 180 °C for 6 h to prepare carbon spheres;
[0054] 2) Prepare a 0.004 g / mL NaCl solution, add polymethyldiallyl ammonium chloride, namely PDDA, to prepare a 0.2 wt.% PDDA solution, add the product obtained in the above step 1) and stir for 60 min before filtering, Obtain carbon spheres-PDDA; prepare 0.004 g / mL NaCl solution, add sodium polystyrene sulfonate, namely PSS, to prepare a 0.1 wt.% PSS solution, add carbon spheres-PDDA and stir for 30 min, then filter to obtain carbon Balls-(PDDA-PSS); adding carbon balls-(PDDA-PSS) to the above PDDA solution to prepare carbon balls-(PDDA-PSS-PDDA) particles, finally making the surface of carbon balls uniformly distributed positive charges;
[0055] 3) Add 0.09 g / mL FeCl 3 ·6H 2 O and 0.04 g / mL CoCl 2 ·6H 2 O dissolved in ethylene glycol, where FeCl 3 ·6H 2 O and CoCl 2 ·6H 2 The molar ratio of O is 2:1; Then urea and poly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com