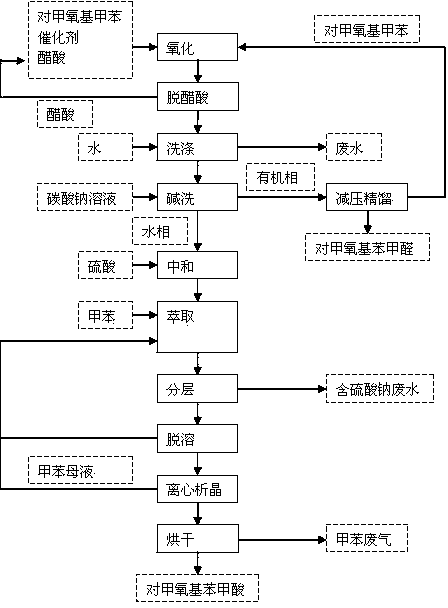
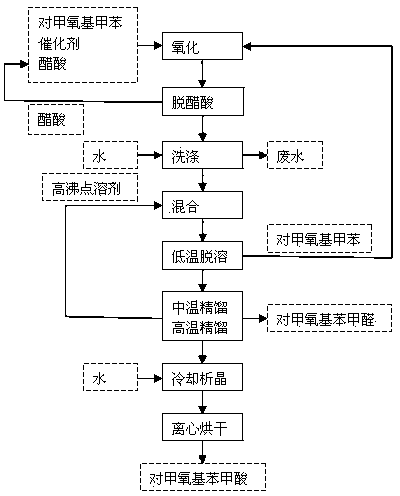
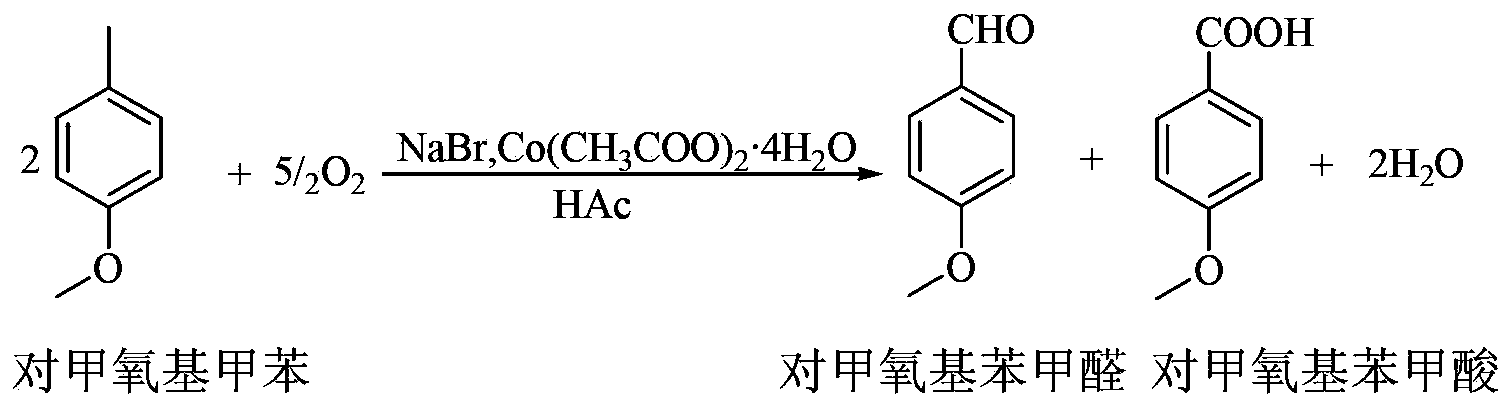
Process for synthesizing p-methoxy benzaldehyde or p-tertbutyl benzaldehyde
A technology for p-methoxybenzaldehyde and p-methoxytoluene, which is applied in the field of substituted toluene oxidation to prepare substituted benzaldehyde, can solve problems such as complicated separation process, and achieve the effects of reducing pollution, reducing discharge and avoiding sodium sulfate-containing wastewater
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] In a 1000mL reaction flask, add 525mL of p-methoxytoluene, 150mL of glacial acetic acid, 50g of cobalt acetate and 30g of sodium bromide, stir, heat to 45°C, and pass oxygen for 4h to complete the reaction; add 800mL of water twice to wash the reaction liquid, to remove catalyst and acetic acid.
[0017] Add 200mL of monomethylbiphenyl to the oil phase after water washing, mix evenly, and desolvate under reduced pressure (-0.09MPa, 85-90℃) to remove p-methoxytoluene; continue to increase the rectification temperature (-0.098MPa, 100 -105°C), gas phase condensation to obtain p-methoxybenzaldehyde (light yellow liquid, acid value: ≤1.0mgKOH / g, content 99.3%); increase the rectification temperature again (-0.098MPa, 118-123°C), distill P-methoxybenzoic acid is discharged, and the gas-phase p-methoxybenzoic acid is passed into the water absorption tower, cooled and crystallized, and p-methoxybenzoic acid (white powder crystals, melting point range 181-185°C) is obtained aft...
Embodiment 2
[0019] In a 1000mL reaction flask, add 700mL of p-tert-butyltoluene, 20mL of glacial acetic acid, 10g of cobalt acetate and 4.5g of sodium bromide, stir, heat to 45°C, and pass oxygen for 4h to complete the reaction; add 1000mL of water twice to wash the reaction liquid , to remove catalyst and acetic acid.
[0020] Add 260mL of biphenyl-biphenyl ether low-melting mixture to the oil phase after water washing, mix well, and carry out desolvation under reduced pressure (-0.09MPa, 80-86°C) to remove excess p-tert-butyltoluene; continue to increase the rectification Temperature (-0.098MPa, 98-104°C), gas phase condensation to obtain p-tert-butylbenzaldehyde (light yellow liquid, content 99.2%); increase the rectification temperature again (-0.098MPa, 114-118°C), remove p-tert-butylbenzaldehyde tert-butylbenzoic acid, p-tert-butylbenzoic acid in the gas phase is passed into the water absorption tower, cooled and crystallized, recrystallized and dried to obtain p-tert-butylbenzoic a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com