Method for synthesizing o-vanillin through utilizing 5-aldehyde vanillin
A technology of aldehyde-based vanillin and ortho-vanillin, which is applied in the preparation of carbon-based compounds, chemical instruments and methods, and the preparation of organic compounds, can solve the problem of high cost, reduce solid waste emissions, and improve economic benefits Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
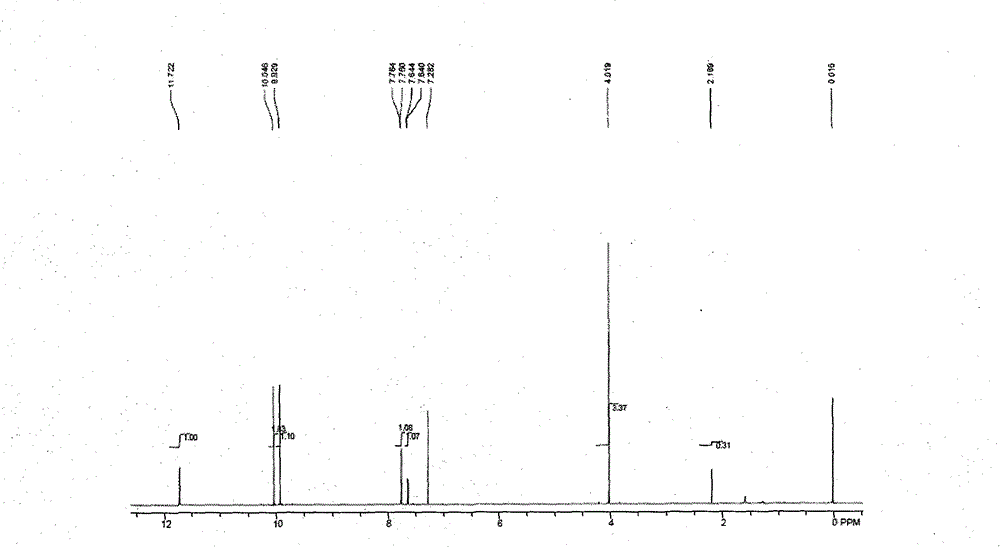
[0017] In the 1000ml glass there-necked flask, add 200 grams of vanillin by-product solid waste, which contains 5-formyl vanillin (nuclear magnetic spectrum see figure 1 ) about 65%, vanillin 10%. Adding ethanol with a mass ratio of 1: 3.5, heating and dissolving, stirring and cooling to crystallize, and obtaining 105 grams of 5-formyl vanillin after filtration and drying, with a purity of 90% (Note: 5-formyl vanillin contains 5% vanillin Lansu), yield 69%. Add the above-mentioned 5-formyl vanillin and 0.11 g of 10% palladium / carbon catalyst into a 250ml three-necked flask equipped with magnetic stirring and a thermometer, heat to dissolve, turn on magnetic stirring and vacuum, adjust the vacuum to 1-4KPa to control the reaction temperature at Reflux at 180-210° C. for 1-2 hours to carry out catalytic dealdehydeization, and 83 grams of the mixture was obtained from the discharge, with an ortho-vanillin content of 72.1% (note: by-product content of 17% vanillin and 10.8% guaia...
Embodiment 2
[0019] Add 300 grams of vanillin by-product solid waste into a 500 ml glass three-necked flask, which contains about 65% of 5-formyl vanillin and about 10% of vanillin. Direct heating and rectification gave 220 g of 5-formyl vanillin with a purity of 87% (note: 5-formyl vanillin contains 13% vanillin), and a yield of 98%. Add the above-mentioned 5-formyl vanillin and 0.22 g of 10% palladium / carbon catalyst into a 250ml three-neck flask equipped with magnetic stirring and a thermometer, heat to dissolve, turn on magnetic stirring and vacuum, adjust the vacuum to 3-6KPa to control the reaction temperature at Reflux at 200-230° C. for 1-2 hours to carry out catalytic dealdehydeization, and 170 grams of the mixture is obtained from the discharge, and the content of ortho vanillin is 70% (note: the content of by-products is 19% vanillin and 10.8% guaiacol), The conversion rate of ortho-vanillin was 62%. After the distillation, an appropriate amount of ethanol is added to dissolve,...
Embodiment 3
[0021] Add 300 grams of vanillin by-product solid waste into a 500 ml glass three-neck flask, which contains about 50% of 5-formyl vanillin and about 20% of vanillin. Direct heating and distillation gave 200 g of 5-formyl vanillin with a purity of 70% (note: 5-formyl vanillin contains 30% vanillin) and a yield of 93%. Add the above-mentioned 5-formyl vanillin and 0.4 g of platinum / carbon catalyst to a 250ml three-necked flask equipped with magnetic stirring and a thermometer, heat to dissolve, turn on magnetic stirring and vacuum, adjust the vacuum to 5-7KPa to control the reaction temperature at 210- Reflux at 250° C. for 1 to 2 hours to carry out catalytic dealdehydeization, and 120 grams of the mixture obtained from the discharge, the content of ortho-vanillin is 53.1% (note: the by-product content of vanillin is 36.5% and guaiacol 10.3%), and the ortho-vanillin content is 53.1%. The pandan conversion rate was 45.5%. After the distillation, an appropriate amount of ethanol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com