Organic amine salt of aminobenzoic acid derivative, and method for producing same
A technology of organic amine salt and manufacturing method, applied in the directions of organic chemistry, organic active ingredients, medical preparations of non-active ingredients, etc., can solve the problems such as no examples, and achieve the effect of excellent stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
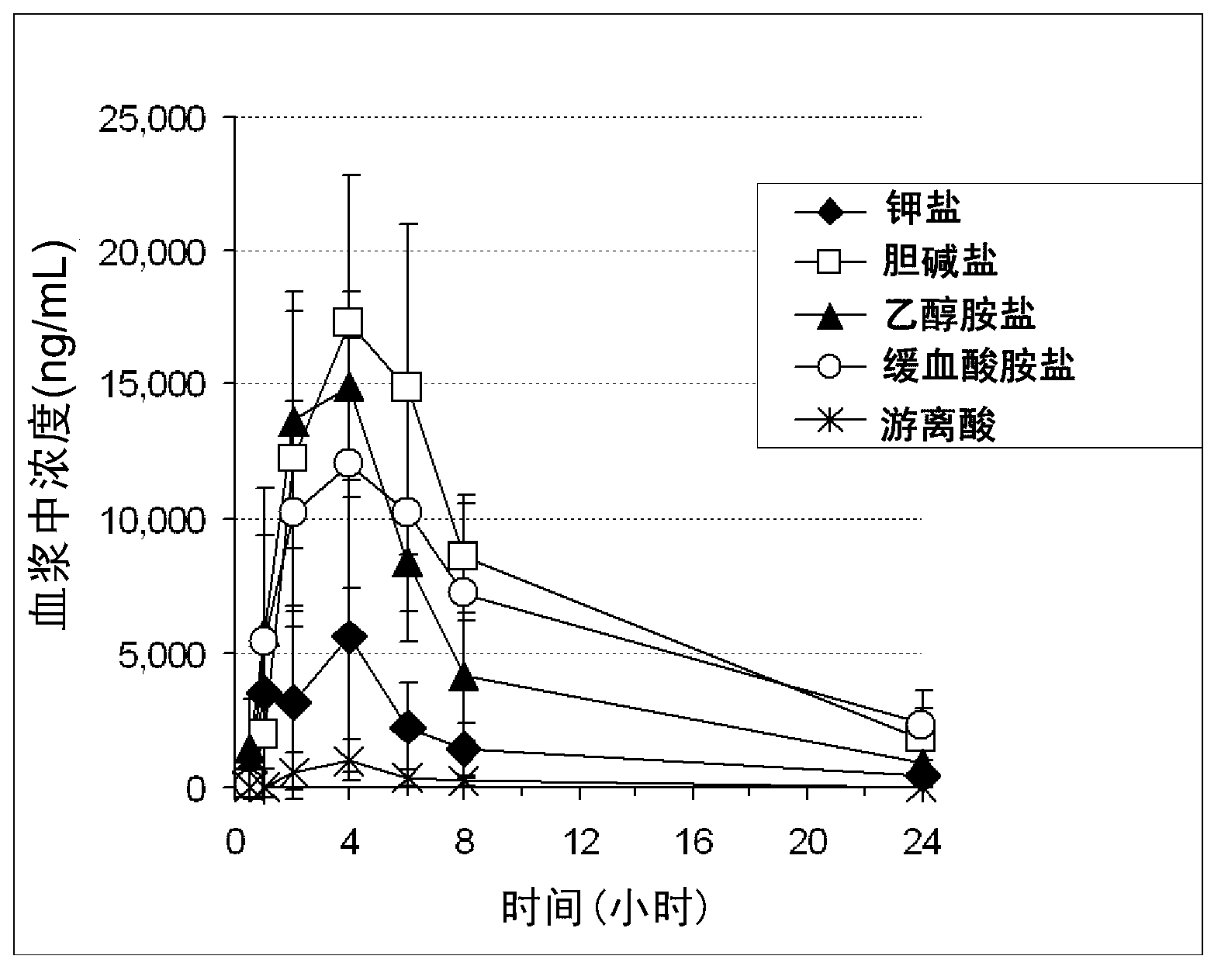
[0102] Hereinafter, the present invention will be described in more detail by showing synthesis examples, test examples, and formulation examples, but the present invention is not limited to these examples.
[0103] In addition, in the embodiments, LC means liquid chromatography, HPLC means high performance liquid chromatography, MS means mass spectrometry, LC / MS means liquid chromatography-mass spectrometry, LC / MS / MS means liquid chromatography-tandem mass spectrometry, TG means thermogravimetric analysis, Cmax means the maximum concentration in plasma, Tmax means the time to reach the maximum concentration in plasma, and AUC means the area under the concentration-time curve in plasma.
[0104] In addition, machine analysis was performed using the following device conditions, respectively.
[0105] 1 H-NMR was measured at 300 MHz.
[0106] TG was measured with TG8120 (manufactured by Rigaku Corporation).
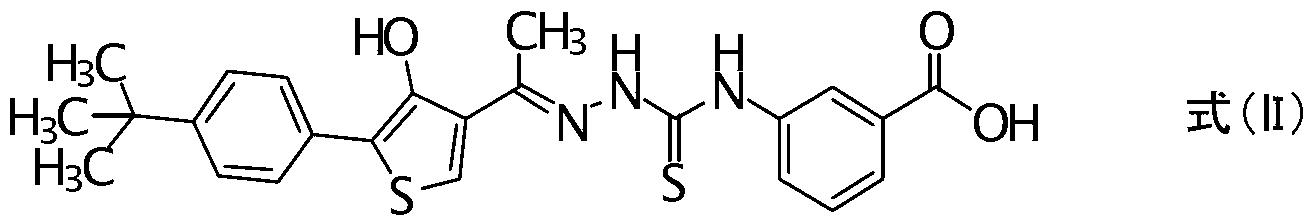
[0107] Reference Synthesis Example 1
[0108] 3-{[((2E)-2-{1-[5-(4-t...
Synthetic example 1
[0114] 3-{[((2E)-2-{1-[5-(4-tert-butylphenyl)-4-hydroxy-3-thienyl]ethylene}hydrazino)thiocarbonyl]amino} Ethanolamine Benzoate.
[0115] In 3-{[((2E)-2-{1-[5-(4-tert-butylphenyl)-4-hydroxy-3-thienyl]ethylidene}hydrazino)thiocarbonyl]amino A methanol solution (24.0 mL) of ethanolamine (1.47 g, 24.07 mmol) was added to an acetonitrile suspension (218 mL) of benzoic acid (10.25 g, 21.92 mmol), and stirred at room temperature for 2 hours and 50 minutes. The generated precipitate was collected by filtration, washed with acetonitrile, and dried under reduced pressure to obtain 10.88 g of the target product (94% yield).
[0116] Appearance: light yellow solid
[0117] 1 H-NMR (300MHz, DMSO-d 6 ):δ1.30(9H,s),2.39(3H,s),2.84(2H,t,J=5.0Hz),3.56(2H,t,J=5.0Hz),7.27(1H,dd,J= 8.0&7.5Hz),7.40(2H,d,J=8.5Hz),7.46(1H,d,J=7.5Hz),7.62(1H,s),7.73(2H,d,J=8.5Hz),7.91 (1H,d,J=8.0Hz),8.50(1H,s)
Synthetic example 2
[0119] 3-{[((2E)-2-{1-[5-(4-tert-butylphenyl)-4-hydroxy-3-thienyl]ethylene}hydrazino)thiocarbonyl]amino} Benzoic acid tromethamine salt
[0120] In 3-{[((2E)-2-{1-[5-(4-tert-butylphenyl)-4-hydroxy-3-thienyl]ethylidene}hydrazino)thiocarbonyl]amino } To a tetrahydrofuran solution (100 mL) of benzoic acid (9.4 g, 20.1 mmol) was added tromethamine (2.7 g, 22.3 mmol) aqueous solution (20 mL), followed by acetonitrile (400 mL), and stirred at room temperature for 1 day. The generated precipitate was collected by filtration and dried under reduced pressure to obtain 11.3 g of the target product (95% yield).
[0121] Appearance: white to light yellow-green solid
[0122] 1 H-NMR (300MHz, DMSO-d 6 ):δ1.30(9H,s),2.38(3H,s),3.45(6H,s),7.29(1H,dd,J=8.0&7.5Hz),7.40(2H,d,J=8.5Hz) ,7.47(1H,d,J=7.5Hz),7.64(1H,s),7.72(2H,d,J=8.5Hz),7.89(1H,d,J=8.0Hz),8.51(1H,s)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com