Fe-doped TiO2 nanotube photocatalyst, and preparation method and application thereof
A technology of photocatalysts and nanotubes, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve complex preparation processes and processes, small specific surface area, nanometer No secondary pollution, large specific surface area, and many adsorption sites can be achieved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Weigh 1.0g P-25TiO 2 powder and 0.0505g of Fe(NO 3 ) 3 9H 2 O, added to 16.0mL of 10mol / L NaOH solution, magnetically stirred for 0.5h, transferred to a polytetrafluoroethylene beaker, reacted at 105°C for 24h, removed and cooled to room temperature, washed with distilled water until neutral. Soak in 0.1mol / L hydrochloric acid for 0.5h, wash again until neutral, dry at 60°C, calcinate in a muffle furnace at 550°C for 2h, and grind to obtain Fe-doped nanotube TiO with an atomic doping amount of 1%. 2 catalyst. The above P-25TiO 2 Powder was purchased from Degussa Corporation, New Jersey. The TiO 2 Powder specific surface area is 50m 2 / g, the average particle size is 21nm, anatase and rutile account for 80% and 20% of the total mass respectively.
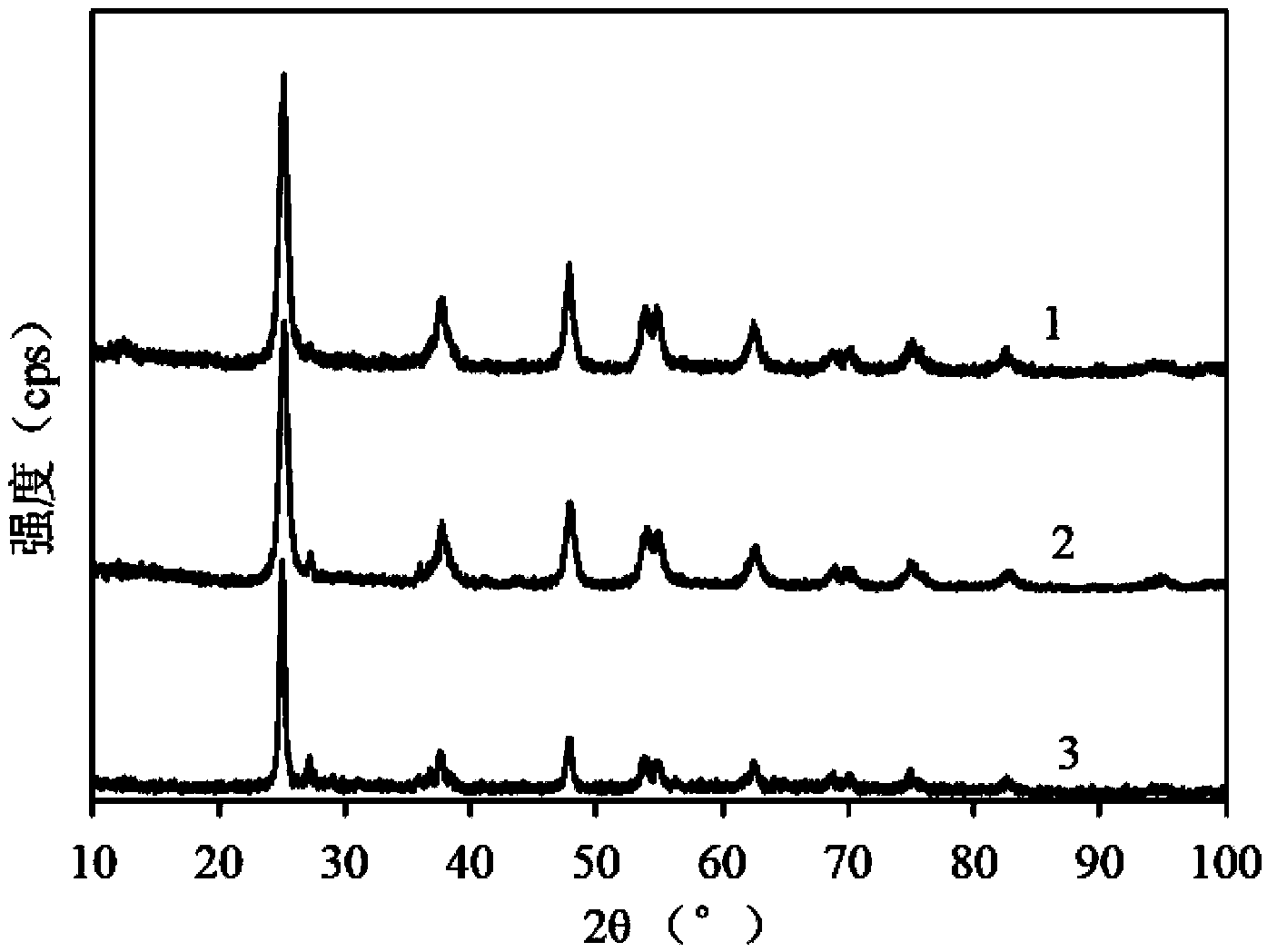
[0034] Analysis of calcined Fe-doped nanotube TiO at 550°C using Rigaku Dmax-RB rotating anode diffractometer (X-ray diffraction, XRD) 2 Crystal phase (Cu Kα target, λ=0.1506nm); ( figure 1 ), the results show that t...
Embodiment 2
[0037] Weigh 1.0g P-25TiO 2 powder and 0.0505g of Fe(NO 3 ) 3 9H2 O, added to 16.0mL of 10mol / L NaOH solution, magnetically stirred for 0.5h, transferred to a polytetrafluoroethylene beaker, reacted at 105°C for 24h, removed and cooled to room temperature, washed with distilled water until neutral. Soak in 0.1mol / L hydrochloric acid for 0.5h, wash again until neutral, dry at 60°C, calcinate in a muffle furnace at 450°C for 2h, and grind to obtain Fe-doped nanotube TiO with an atomic doping amount of 1%. 2 catalyst. The above P-25TiO 2 Powder was purchased from Degussa Corporation, New Jersey. The TiO 2 Powder specific surface area is 50m 2 / g, the average particle size is 21nm, anatase and rutile account for 80% and 20% of the total mass respectively.
[0038] Analysis of calcined Fe-doped nanotube TiO at 450°C using Rigaku Dmax-RB rotating anode diffractometer (X-ray diffraction, XRD) 2 Crystal phase (Cu Kα target, λ=0.1506nm); ( figure 1 ), the results show that the...
Embodiment 3
[0041] Weigh 1.0g P-25TiO 2 powder and 0.0505g of Fe(NO 3 ) 3 9H 2 O, added to 16.0mL of 10mol / L NaOH solution, magnetically stirred for 0.5h, transferred to a polytetrafluoroethylene beaker, reacted at 105°C for 24h, removed and cooled to room temperature, washed with distilled water until neutral. Soak in 0.1mol / L hydrochloric acid for 0.5h, wash again until neutral, dry at 60°C, calcinate in a muffle furnace at 500°C for 2h, and grind to obtain Fe-doped nanotube TiO with an atomic doping amount of 1%. 2 catalyst. The above P-25TiO 2 Powder was purchased from Degussa Corporation, New Jersey. The TiO 2 Powder specific surface area is 50m 2 / g, the average particle size is 21nm, anatase and rutile account for 80% and 20% of the total mass respectively.
[0042] Analysis of calcined Fe-doped nanotube TiO at 500℃ using Rigaku Dmax-RB rotating anode diffractometer (X-ray diffraction, XRD) 2 Crystal phase (Cu Kα target, λ=0.1506nm); ( figure 1 ), the results show that th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com