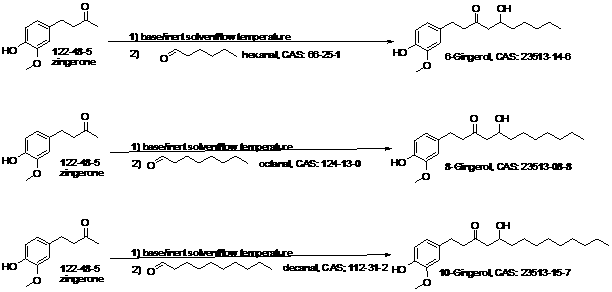
Chemical synthetic process of gingerols
A technology of gingerol and process method, applied in the field of innovative drug preparation and process, to achieve the effects of less discharge, high yield and mild process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The synthesis of embodiment 1,6-gingerol:
[0026] Add 40 grams of gingerone and 2 liters of dry tetrahydrofuran to a 5-liter four-necked bottle. Add an electric stirrer, a low temperature thermometer and a dropping funnel to the flask, and replace it with dry nitrogen several times. Soak the flask in a dry ice-acetone bath, and when the temperature of the solution in the flask drops to -60°C, slowly add a mixture of n-hexanal (40 ml) and 500 ml tetrahydrofuran through a dropping funnel. The dropwise addition process takes 1 hour. After the dropwise addition was completed, the reaction temperature was slowly raised to -40°C and stirred for 5 hours. 100 ml of methanol was added to the reaction solution. The reaction mixture was concentrated in an ice bath at 0°C. The obtained oil was separated by silica gel column chromatography. The mobile phase is ethyl acetate / petroleum ether=2%~5%. The product was concentrated to a viscous oil. Yield: 52 g, 87%. 1 H-NMR (CDC...
Embodiment 2
[0027] The synthesis of embodiment 2,8-gingerol:
[0028] 8-gingerol was synthesized by a method similar to 6-gingerol. Yield: 72%; 1 H-NMR (CDCl 3 , 500 MHz)δ 0.87 (s, 3H), 1.26 (m, 12H), 2.50 (m, 2H), 2.78 (m, 4H), 2.99 (brs, 1H), 3.88 (s, 3H), 4.02 (m , 1H), 5.59 (s, 1H), 6.65 (m, 2H), 6.82 (d, 1H, J = 8.0 Hz).
Embodiment 3
[0029] The synthesis of embodiment 3,10-gingerol:
[0030] 10-gingerol was synthesized similarly to 6-gingerol. Yield: 75%; 1H-NMR (CDCl 3 , 500 MHz) δ 0.87 (s, 3H), 1.26 (m, 16H), 2.51 (m, 2H), 2.78 (m, 4H), 3.12 (brs, 1H), 3.88 (s, 3H), 4.02 (m , 1H), 5.55 (s, 1H), 6.65 (m, 2H), 6.82 (d, 1H, J = 8.0 Hz).
[0031]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com