Method for identifying whether purified thymosin alpha 1 contains deletion peptide or not
A technology for thymosin and missing peptides, which is applied in the field of identification of whether purified thymosin α1 contains missing peptides, quality detection of thymosin α1, can solve the problem that there is no public method for measuring thymosin α1 missing peptides, thymosin α1 is not disclosed, and it is not suitable for clinical practice of thymosin α1 Status and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Solid-phase synthesis of thymosin α1
[0025] 1. Preparation of Fmoc-Asn(Trt)-Wang Resin
[0026] Weigh 10g (3.1mmo1) of Wang Resin and place it in a solid-phase reaction synthesis column. Add 60ml of DCM to swell the resin for 10 minutes, and then remove the DCM. Wash the resin with DMF three times, 50ml each time, blow air for two minutes, and remove the solvent. Weigh 5.5g (9.3mmo1) Fmoc-Asn(Trt) and 1.38g (10.23mmol) HOBt in a ground flask, add 50ml DMF to dissolve. Add 2.15ml (13.95mmol) DIC to activate 5min under cooling in an ice water bath. The activated amino acid was added to the resin washed with DMF, and then 0.23 g (1.86 mmol) of DMAP was added, and the mixture was stirred with nitrogen gas, and reacted at room temperature for 2-4 hours. After the reaction, the resin was washed three times with DMF, 50 ml each time, blowing air for two minutes, and draining the solvent. Take a small amount of resin, shrink it three times with methanol, dry it in v...
Embodiment 2
[0049] Example 2 Purification of thymosin α1 by solid phase synthesis
[0050] 1. One-time purification of thymosin α1 by solid phase synthesis
[0051] Waters 2487 detector, Waters 600 pump, Dima C 18 Preparation column (10μm, 250×21.2mm), ultraviolet detector, wavelength 215nm, TFA / acetonitrile / water gradient elution, the elution gradient is listed in Table 1.
[0052] Table 1 Elution gradient of one-time purification of crude thymosin α1
[0053]
[0054] Dissolve 6.27 g of solid-phase synthetic thymosin α1 crude product in 250 ml of water, first adjust pH 8 with ammonia, then adjust pH 5 with acetic acid, filter, and apply the filtrate to the column. According to the gradient elution in Table 1, the fractions were collected, freeze-dried, and the obtained solid was subjected to secondary purification.
[0055] 2. Secondary purification of thymosin α1 by solid phase synthesis
[0056] Waters 2487 detector, Waters 600 pump, Dima C 18 Preparative column (10μm, 250×21.2mm), UV detector,...
Embodiment 3
[0060] Example 3 Identification of missing peptide map after secondary purification of thymosin α1 by solid phase synthesis
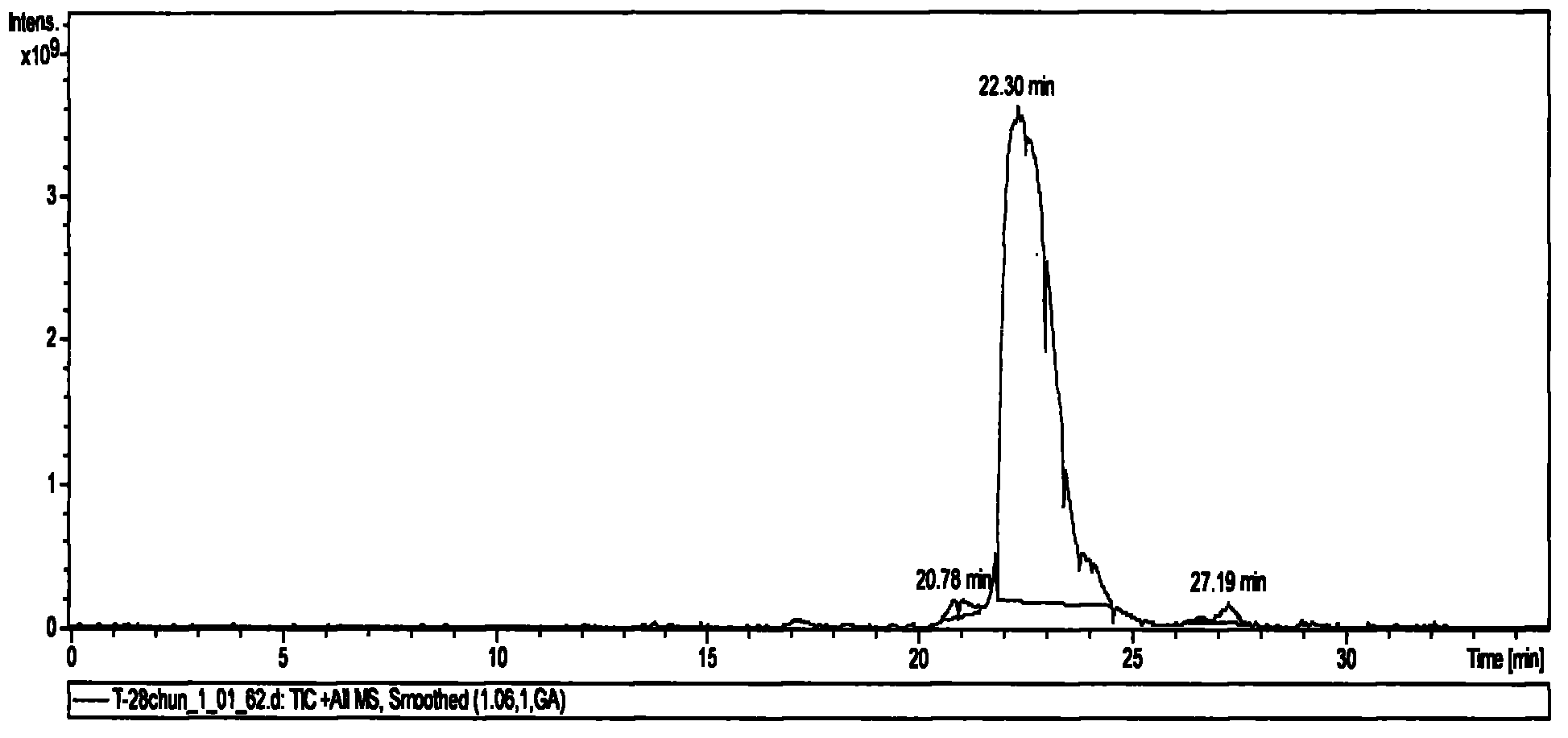
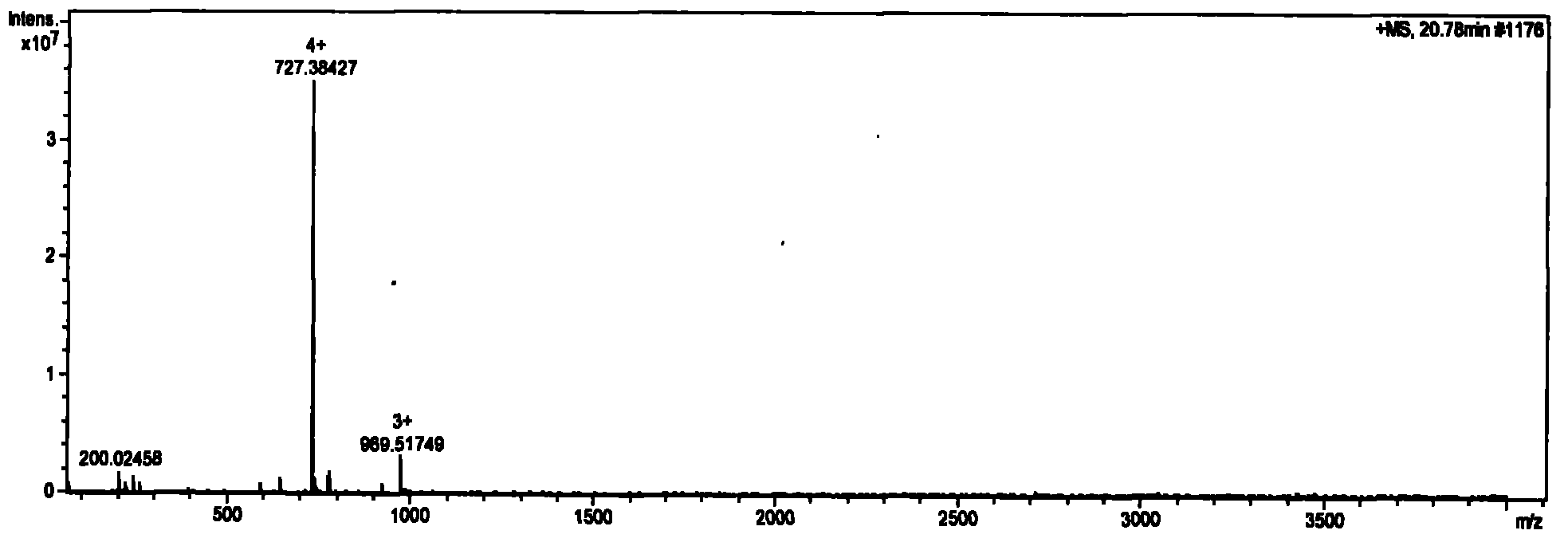
[0061] Take a small amount of solid-phase synthetic thymosin α1 after secondary purification to obtain pure product in LC (Agilent 1200 automatic sampling, Waters Xterra RP18 analytical column, 5μm, 3.0×150mm) / MS (Bruker Solatix FT-ICR-MS, ion current detection Analyze on the combined instrument. The gradient was eluted with 0.1% formic acid / acetonitrile, and the elution gradient is listed in Table 3. Ion current spectrum see figure 1 . figure 1 It consists of 3 peaks, which appear at 20.78min, 22.30min and 27.19min respectively. Their peak areas are 0.36%, 99.16% and 0.47%, respectively.
[0062] Table 3 The elution gradient of pure thymosin α1
[0063]
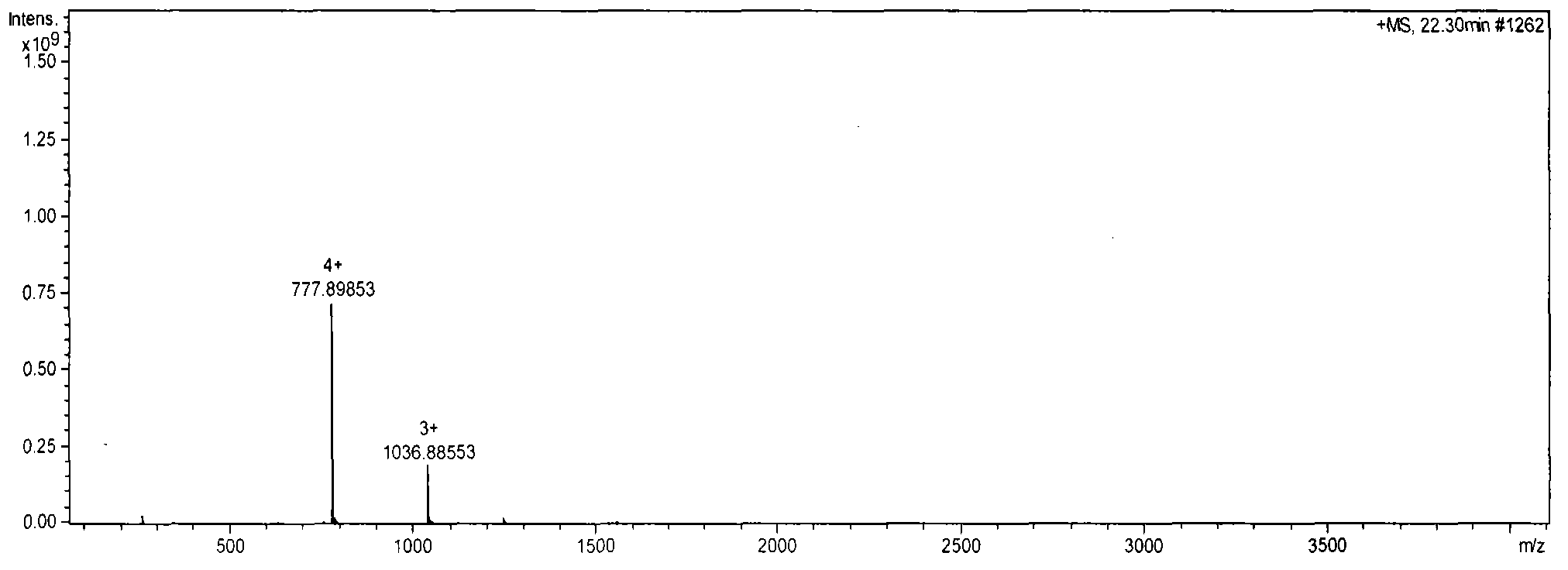
[0064] The corresponding mass spectrum of the peak appearing at 22.30 min is shown in figure 2 , The mass of the ion is 1036.88553×3, which is thymosin α1
[0065] Ac-Ser-Asp-Ala-Ala-Val-Asp-Thr-Ser-Ser-G...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com