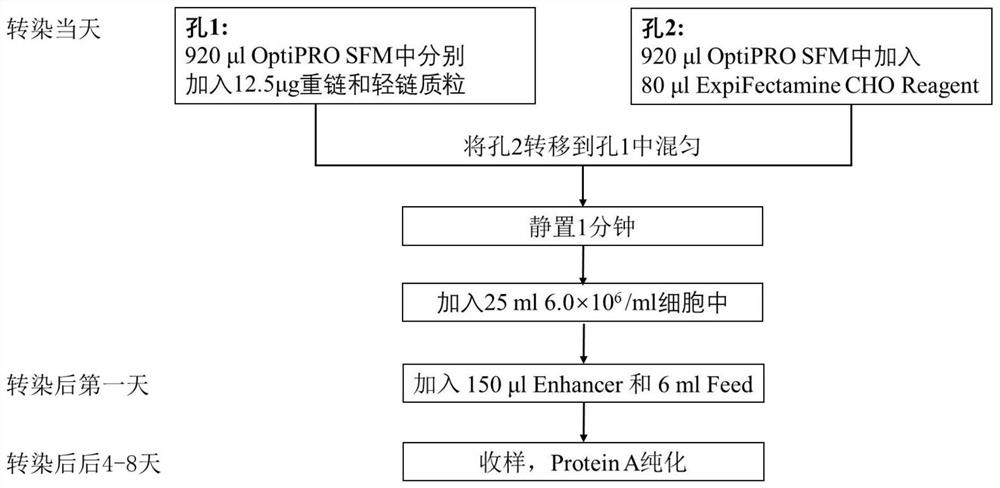
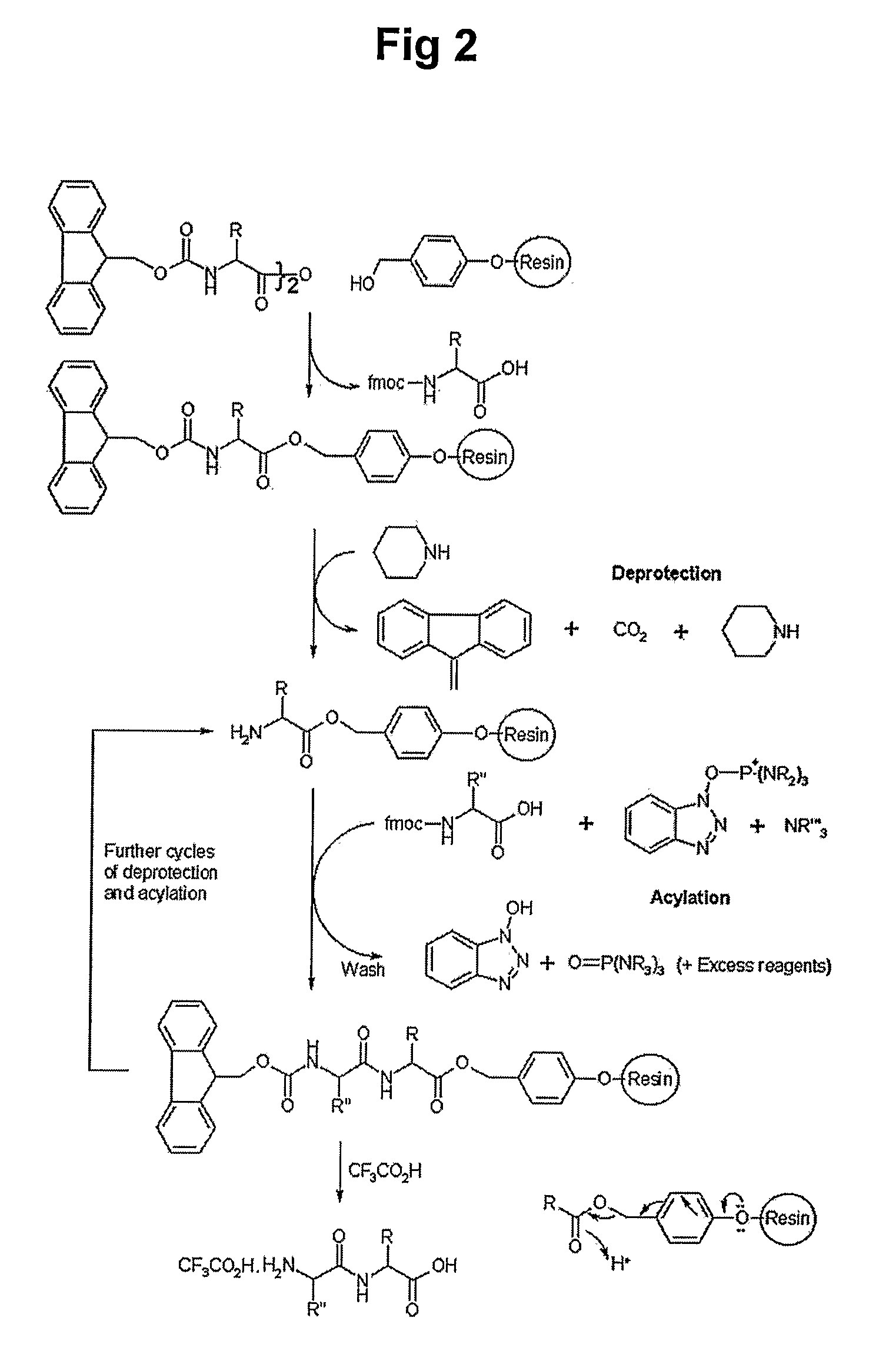
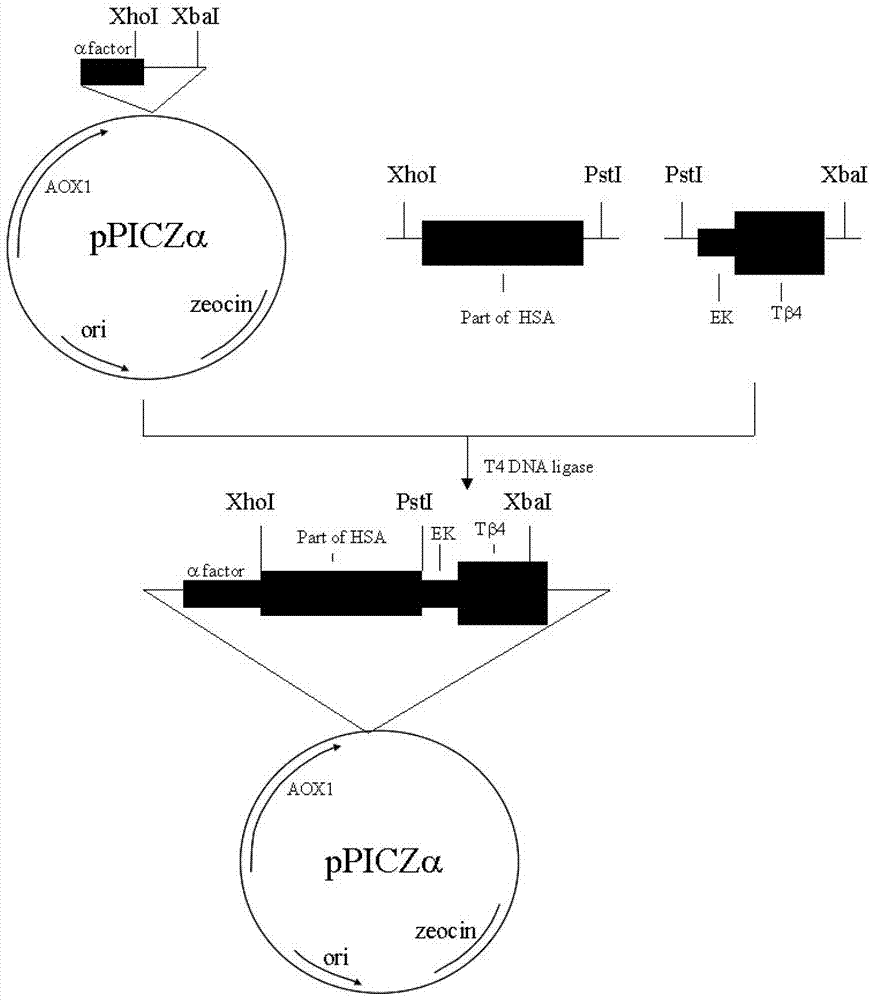
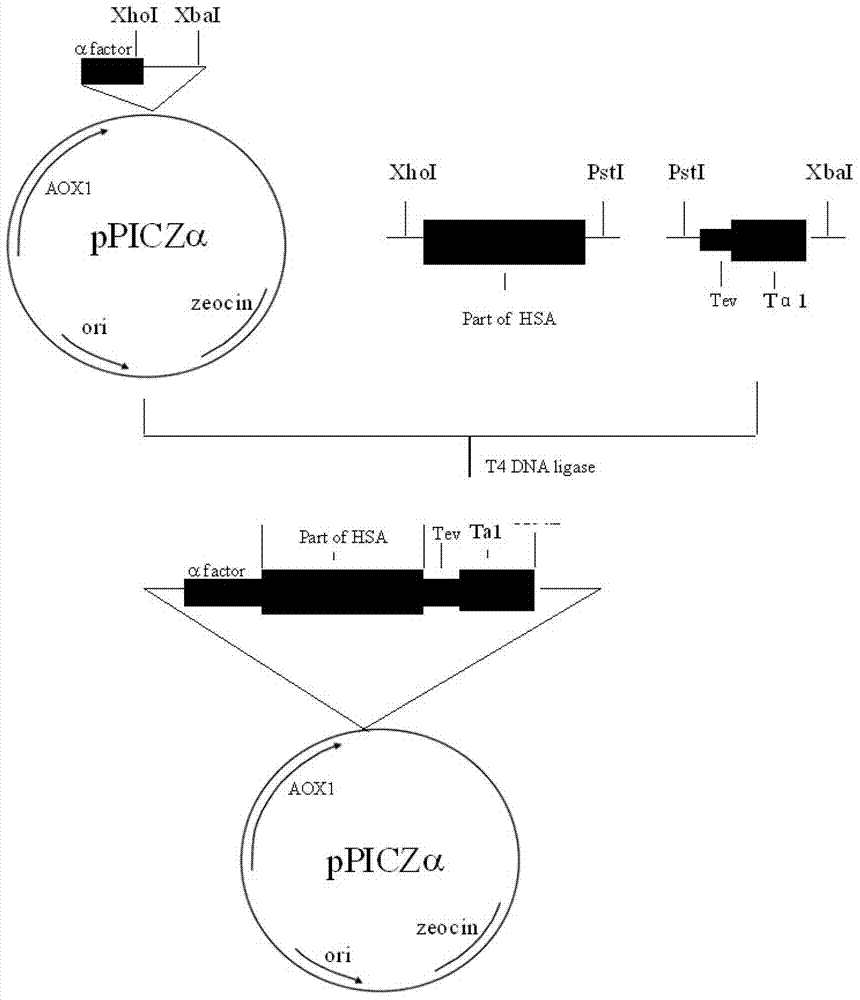
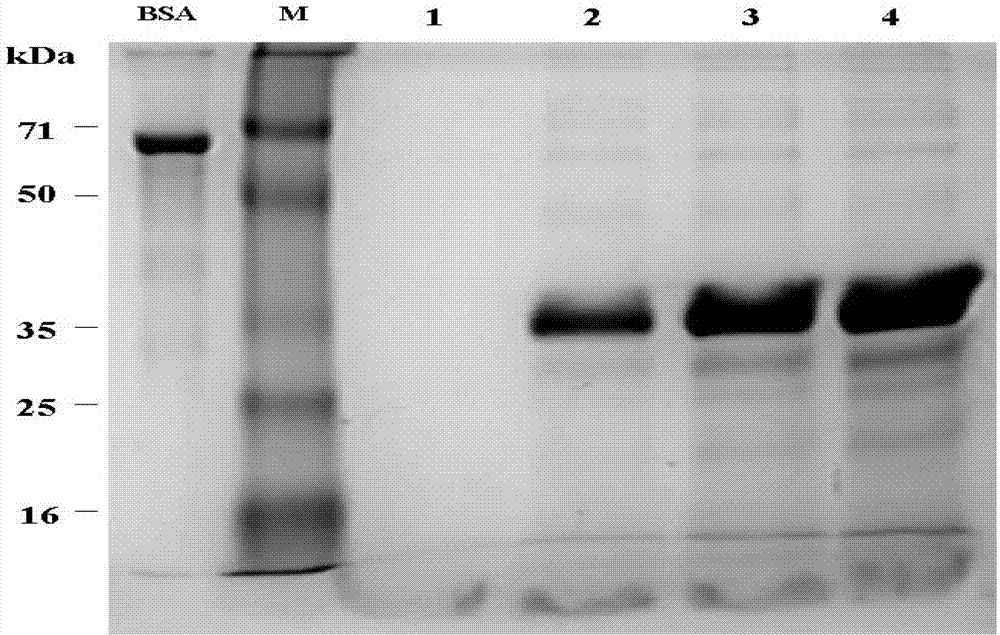
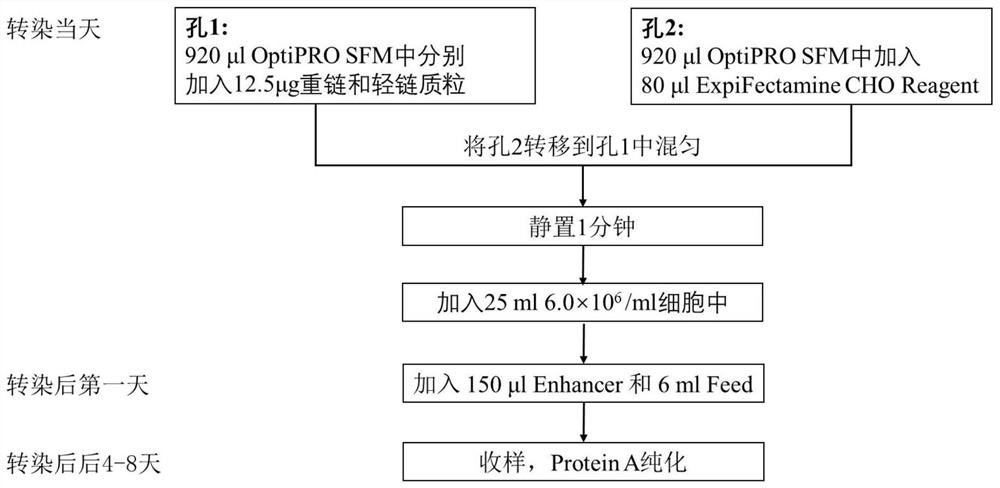
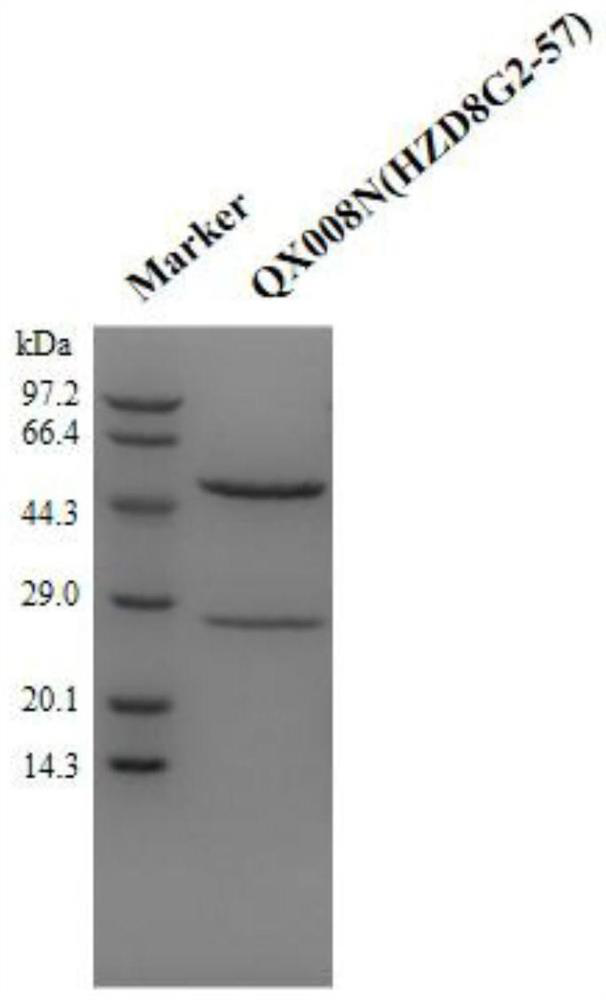
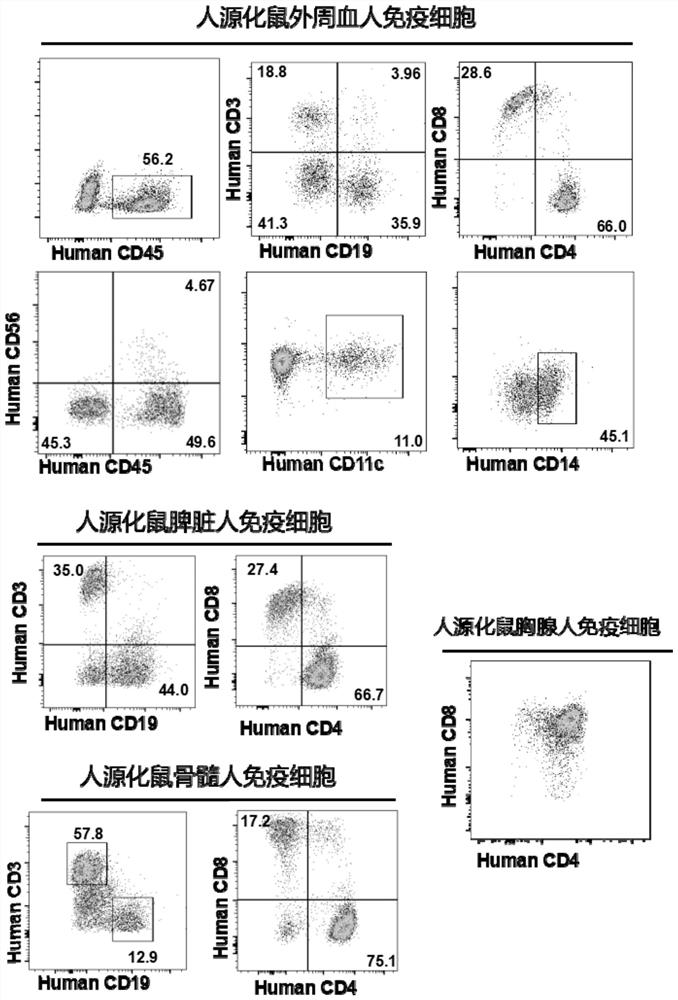
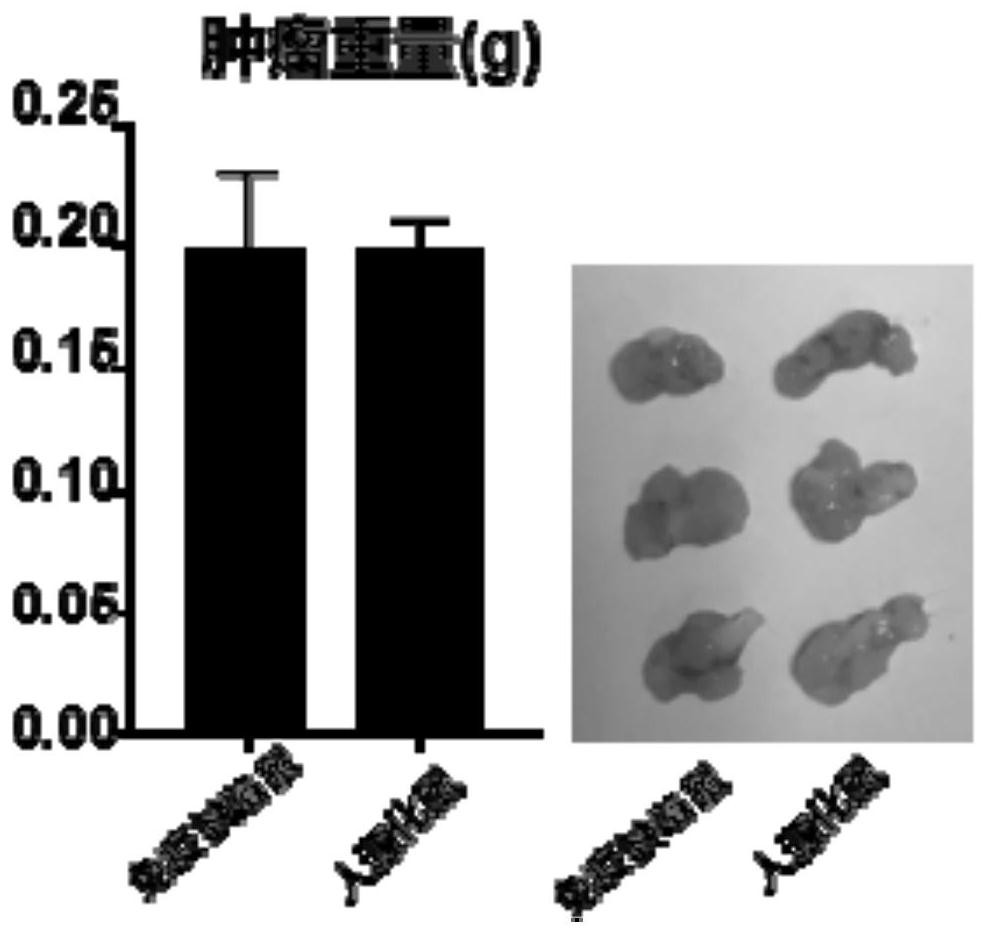
Patents
Literature
37 results about "HUMAN THYMUS" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The thymus was a gland located in the upper chest of a Human body. It played an important role in the Human immune system.
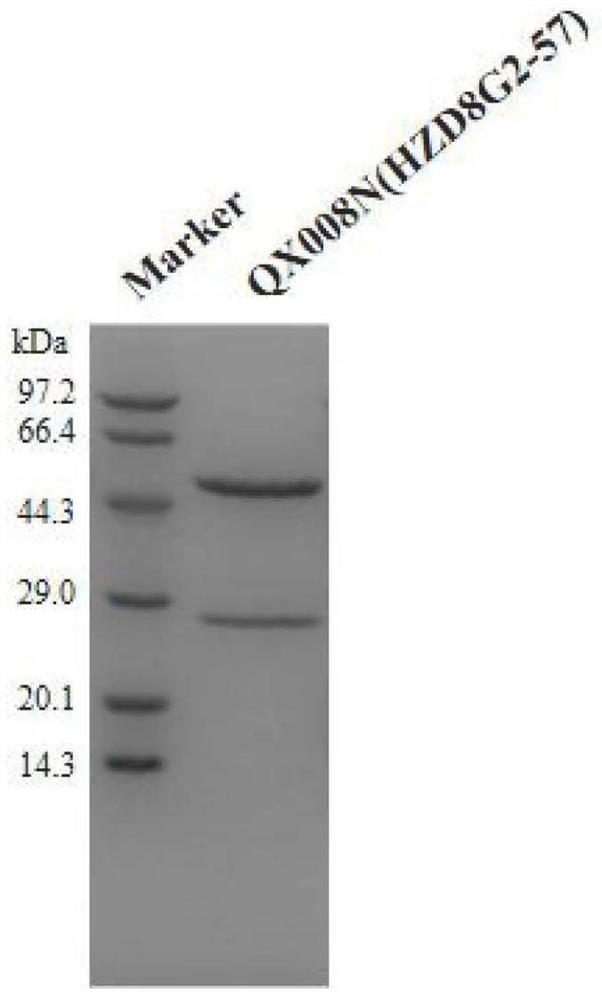
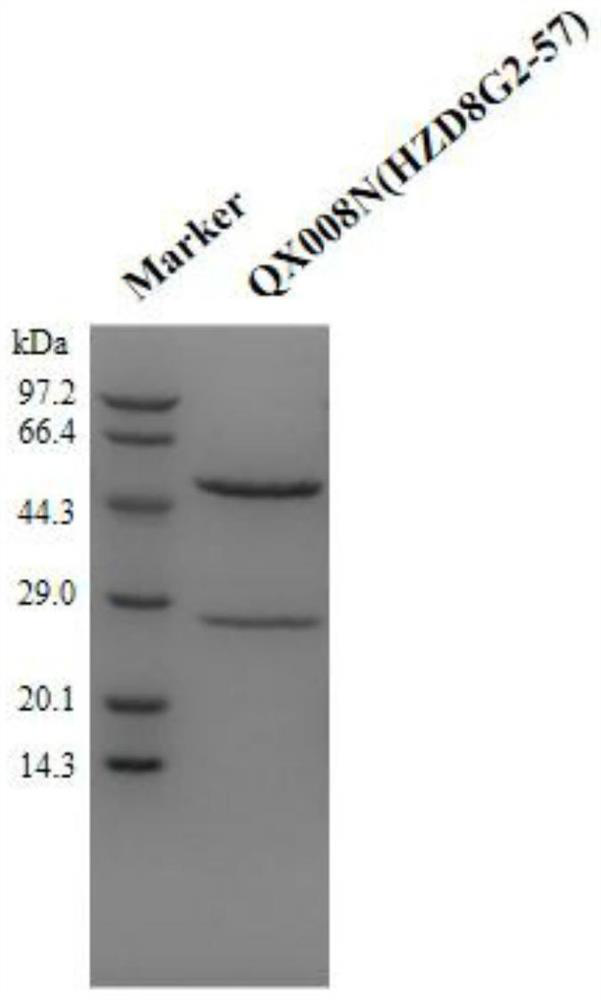
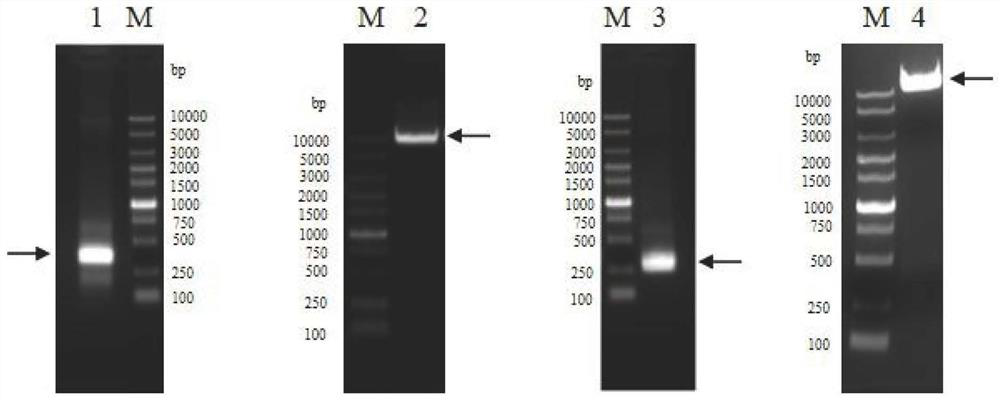
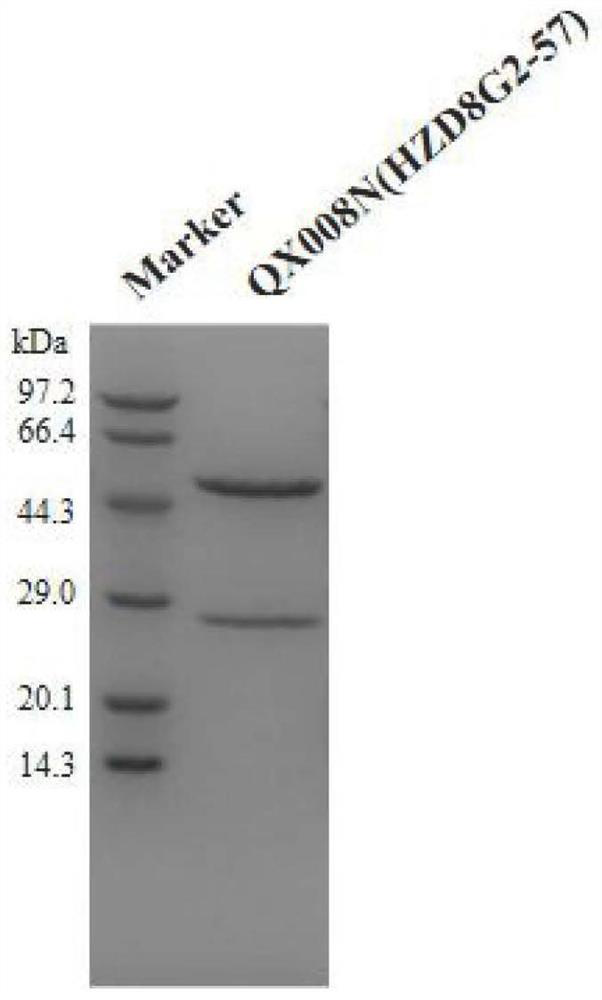
Human thymus stromal lymphopoietin monoclonal antibody and application thereof
ActiveCN111196850AHigh affinityInhibition of activationAntipyreticAnalgesicsAllergic dermatitisEsophagitis
The invention relates to the technical field of antibodies, and especially relates to a human thymus stromal lymphopoietin monoclonal antibody and an application thereof. The TSLP antibody can be specifically combined with hTSLP, has high affinity, and can effectively inhibit the combination of TSLP and a receptor thereof so as to inhibit activation of a downstream signal path and activation of immune cells by TSLP. The TSLP antibody has great significance in detection of the content of hTSLP and allergic asthma, and diagnosis, treatment and prognosis of inflammatory or allergic inflammatory diseases such as chronic obstructive pulmonary disease, allergic dermatitis, allergic rhinitis, allergic nasosinusitis, eosinophilic granulocytic esophagitis, allergic conjunctivitis, inflammatory bowel disease or atopic dermatitis.
Owner:IPHASE THERAPEUTICS LTD
Kit for detecting human immune ages and application of kit
InactiveCN109490544AEvaluate immunityRapid Quantitative AnalysisIndividual particle analysisBiological testingFluoresceinMonoclonal antibody
The invention provides a kit for detecting human immune ages and an application of the kit. Reagents in the kit comprise CD3, CD4, CD31 and CD45RA monoclonal antibodies which are four fluoresceins applicable to flow cytometers, and the colors of the four fluoresceins are different. The reagents in the kit comprise, in weight percent, 0.66-94.34% of fluorescent labeled CD3, 0.66-94.34% of fluorescent labeled CD4, 0.66-94.34% of fluorescent labeled CD31 and 0.66-94.34% of fluorescent labeled CD345RA. The kit is used for detecting recently outputted cell number of human thymuses, the immune agesof people are determined according to detection results of the recently outputted cell number of the human thymuses, and the immune ability of a human body is evaluated.
Owner:ZHEJIANG BOZHEN BIOTECH CO LTD
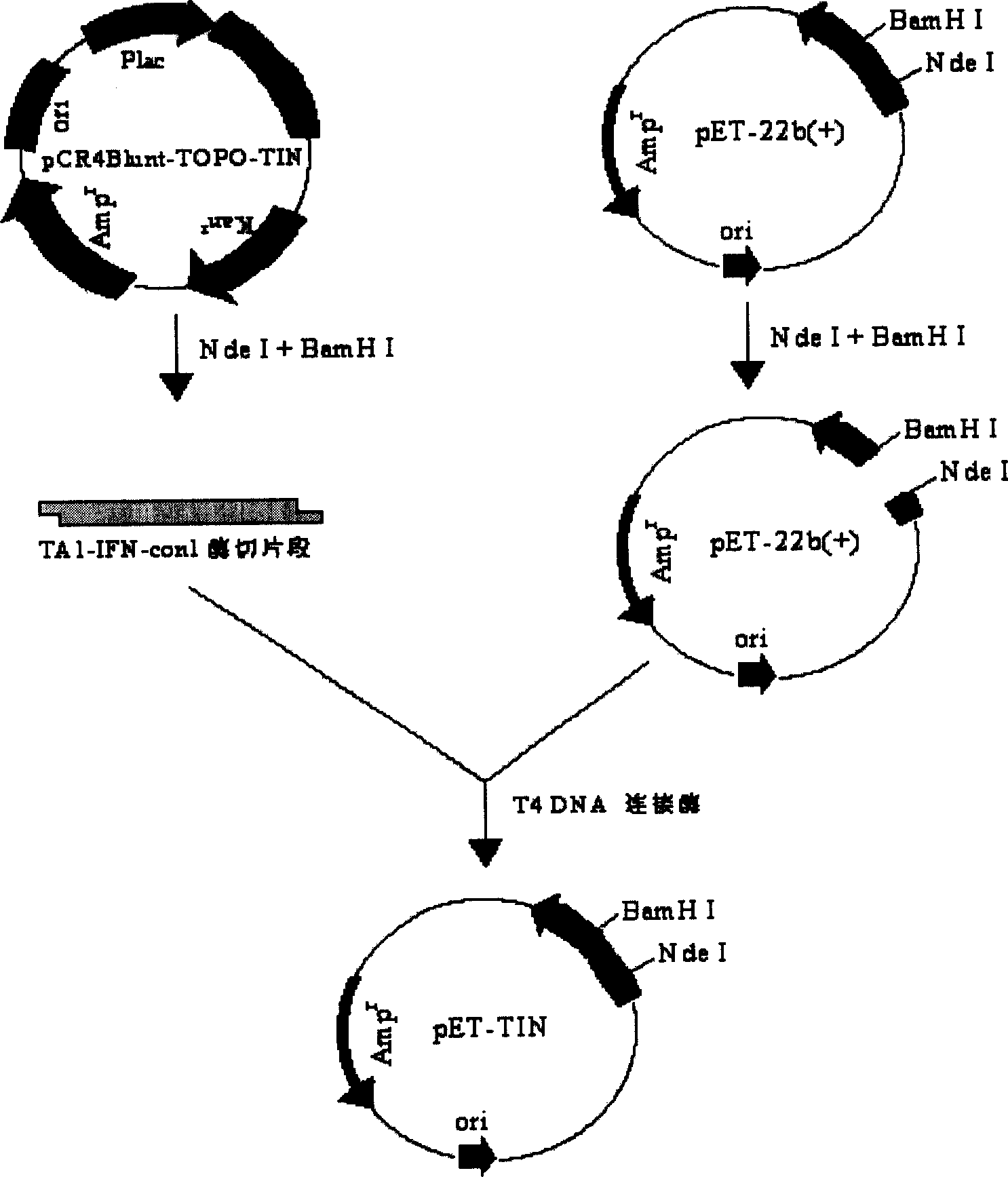
Fusion protein of human thymosin alpha1 and human composite interferon and preparation thereof
InactiveCN1523040ASimple purification processStrong antiviral activityPeptide/protein ingredientsAntiviralsPurification methodsVirus
The present invention relates to a fusion protein of human thymosin alpha 1and human compound interferon, and said protein expression and purification method and application of said protein which can be used as medicine for resisting virus and resisting tumor.
Owner:重庆康尔威药业股份有限公司
Method of preparing natural human thymosin a1 using series expression mode
ActiveCN1724663ASimple processSimple and efficient operationFermentationVector-based foreign material introductionInclusion bodiesEnzyme
The invention discloses a method to manufacture biology polypeptide, especially a method to make natural human thymosin by gene series express method. It contains compounding two polynucleotide section, compounding double enzyme cut, T alpha 1 gene three times connecting in series, constructing high efficiency expression, constructing engineering fungus, expressing T alpha 1 polypeptide six series bodies in engineering fungus, purifying and cracking. The invention could abundantly express aim albumen, and it has simple technology, easy to operate and low cost.
Owner:广东暨大基因药物工程研究中心有限公司
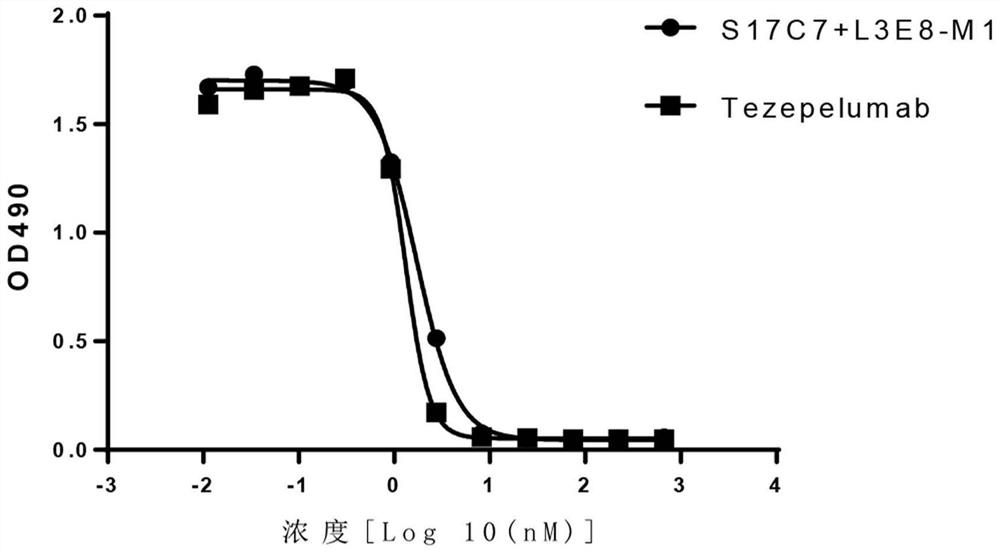
Anti-human TSLP monoclonal antibody and application thereof
ActiveCN113683694AGood neutralizing activityQuite affinitySenses disorderDigestive systemDiseaseComplementarity determining region
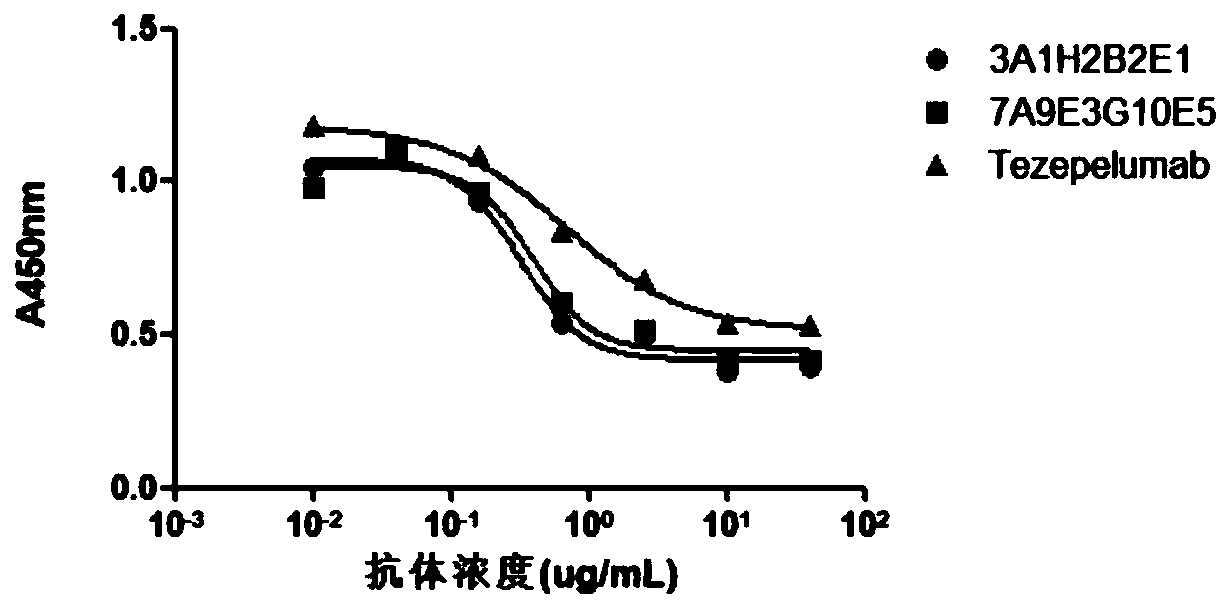
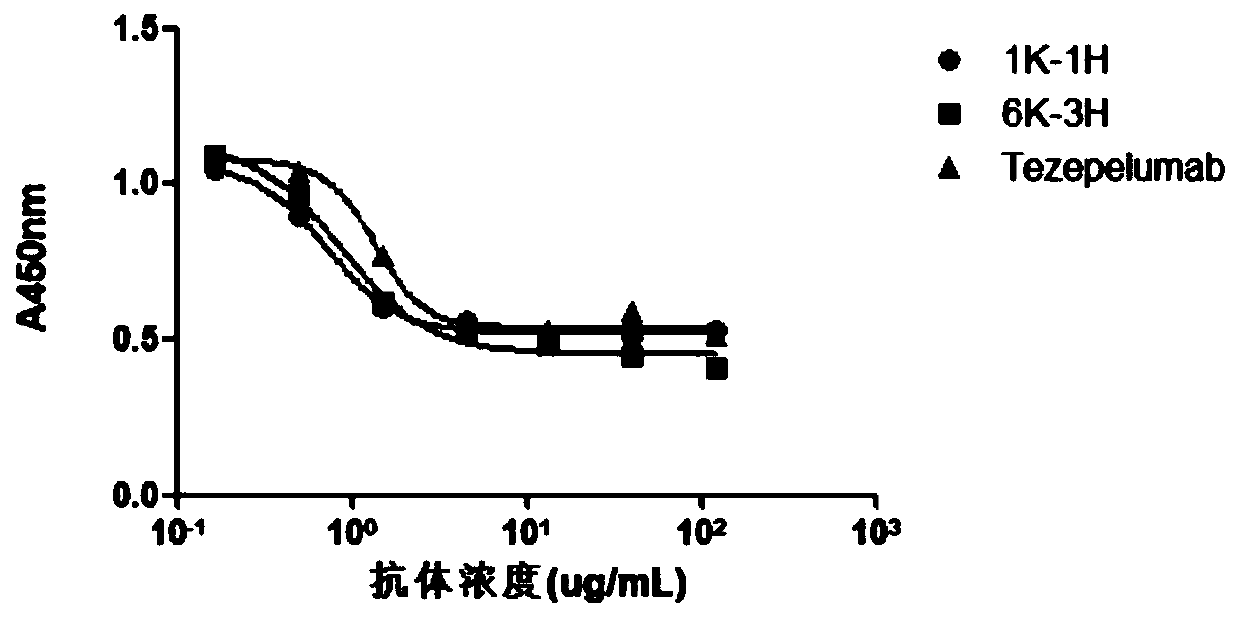
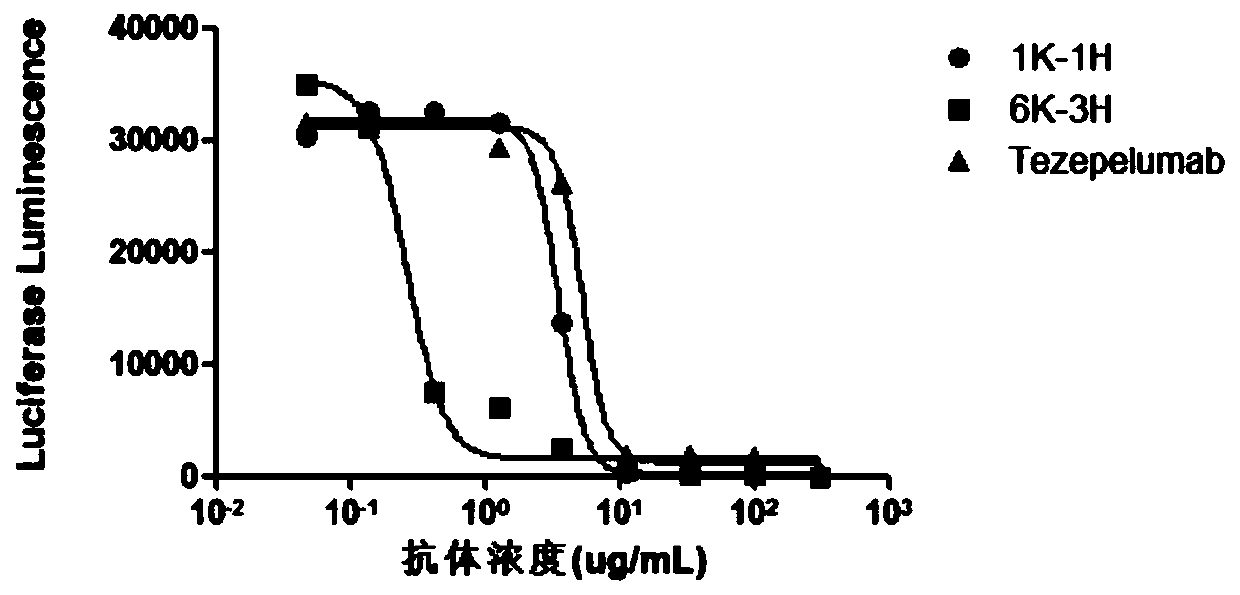
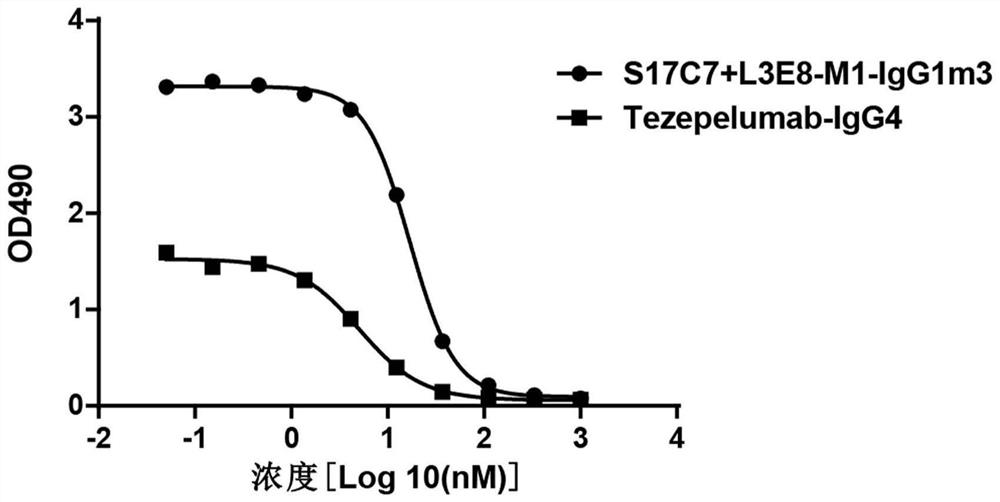
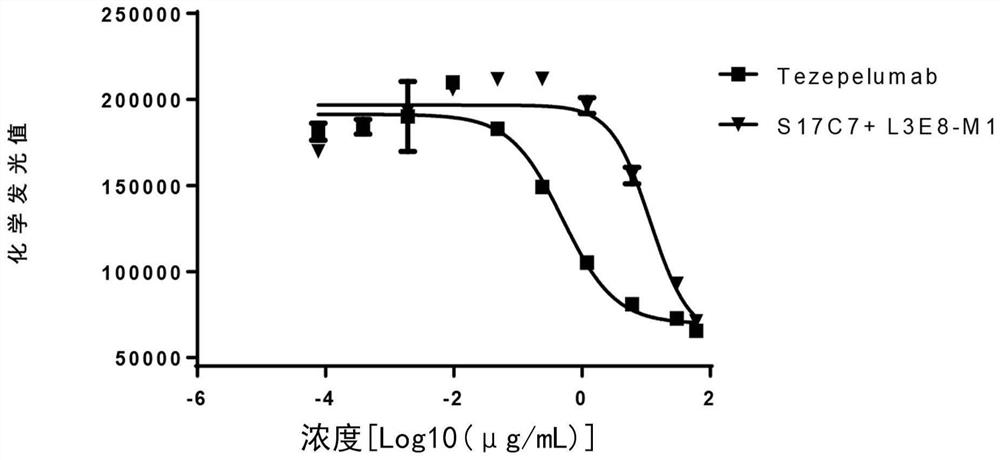
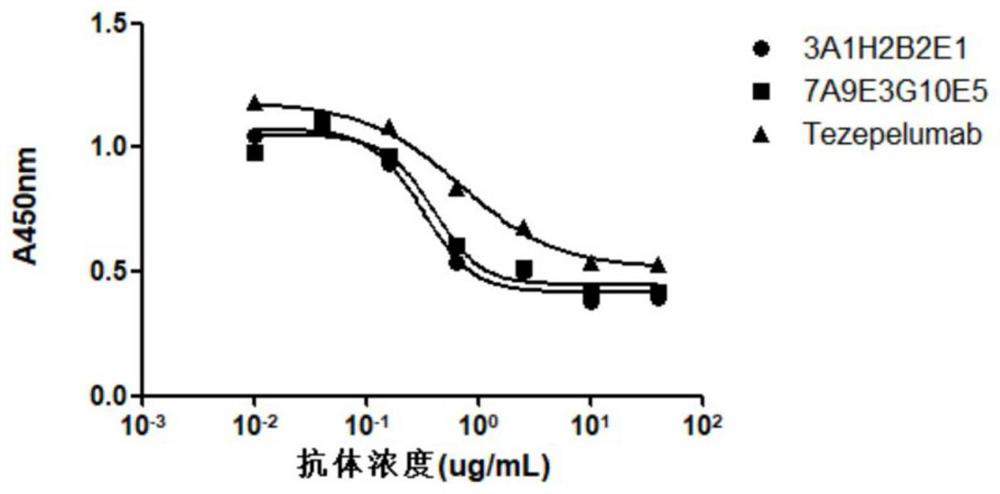
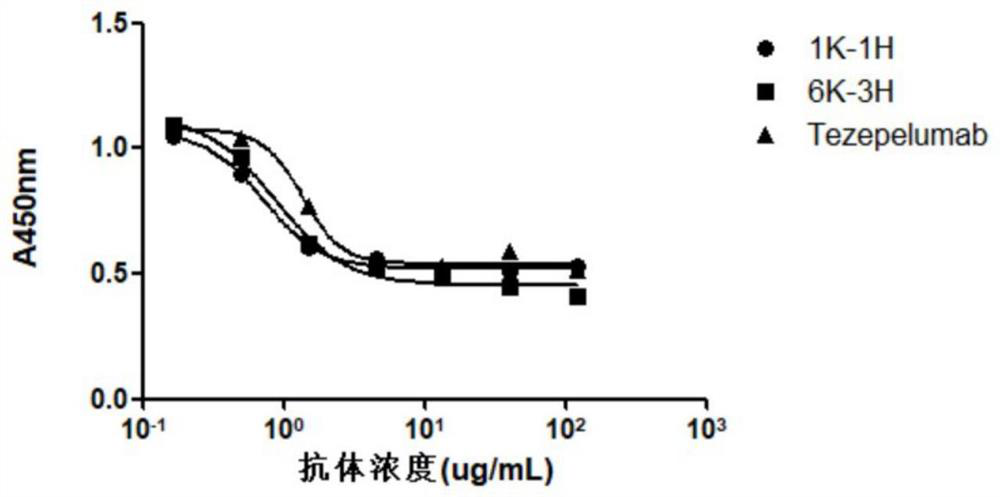
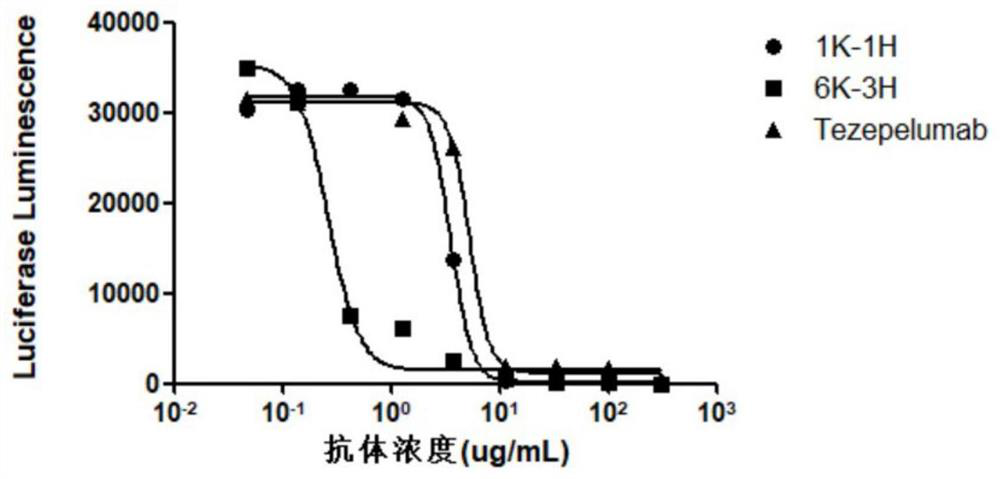
The invention provides an anti-human thymic stromal lymphopoietin (TSLP) monoclonal antibody and an application thereof. The anti-human thymic stromal lymphopoietin (TSLP) monoclonal antibody comprises three heavy chain complementary determining regions (CDR-H1, CDR-H2 and CDR-H3) and three light chain complementary determining regions (CDR-L1, CDR-L2 and CDR-L3), and (a) the amino acid sequence of the CDR-H1 is as shown in SEQ ID NO: 1, and (b) the amino acid sequence of CDR-H2 is as shown in SEQ ID NO: 2; (c) the amino acid sequence of the CDR-H3 is as shown in SEQ ID NO: 3; (d) the amino acid sequence of the CDR-L1 is as shown in SEQ ID NO: 4; (e) the amino acid sequence of the CDR-L2 is as shown in SEQ ID NO: 5; and the amino acid sequence of the (f) CDR-L3 is as shown in SEQ ID NO: 6. Compared with an anti-human TSLP monoclonal antibody Tezepelumab (prepared according to sequence expression disclosed in the patent), the anti-human TSLP monoclonal antibody has the advantages that the affinity of the anti-human TSLP monoclonal antibody combined with the human TSLP is equivalent, the neutralizing activity of the anti-human TSLP monoclonal antibody at the cellular level is superior to that of the Tezepelumab, and the anti-human TSLP monoclonal antibody is expected to show a good clinical effect in the aspect of preventing and treating related diseases.
Owner:QYUNS THERAPEUTICS CO LTD
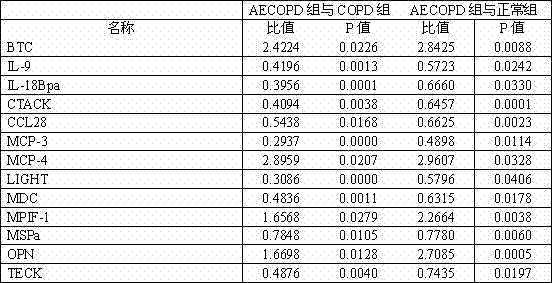
Kit used for diagnosing acute exacerbation period of chronic obstructive pulmonary disease
InactiveCN102253220AEasy to measure and reliableBiological testingInterleukin-18 binding proteinAbnormal macrophage
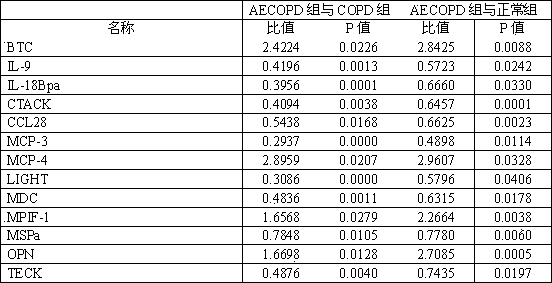
The invention relates to a kit used for diagnosing the acute exacerbation period of chronic obstructive pulmonary disease. The kit comprises a cell factor and an enzyme labeled antibody thereof and is characterized in that the cell factor is interleukin 9, interleukin 18 binding protein A, C-C sequence ligand 28, a human skin T cell capture chemokine, a Beta cytokine, a monocyte chemoattractant protein-3, a monocyte chemoattractant protein-4, a lymphotoxin induced protein entering the T cell, a macrophage-derived chemokine, a human bone marrow suppression factor 1, a human macrophage stimulating protein, an osteopontin and a human thymus expressed chemokine. The invention firstly proposes that the cell factor can be used as a leading indicator for diagnosis of the acute exacerbation period of the chronic obstructive pulmonary disease.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
Multiple antibodies against human TSLP and uses thereof
The invention provides a bispecific antibody against human thymic stromal lymphopoietin (TSLP), a monoclonal antibody and uses of the bispecific antibody and the monoclonal antibody.
Owner:BEIJING WISDOMAB BIOTECHNOLOGY CO LTD +2
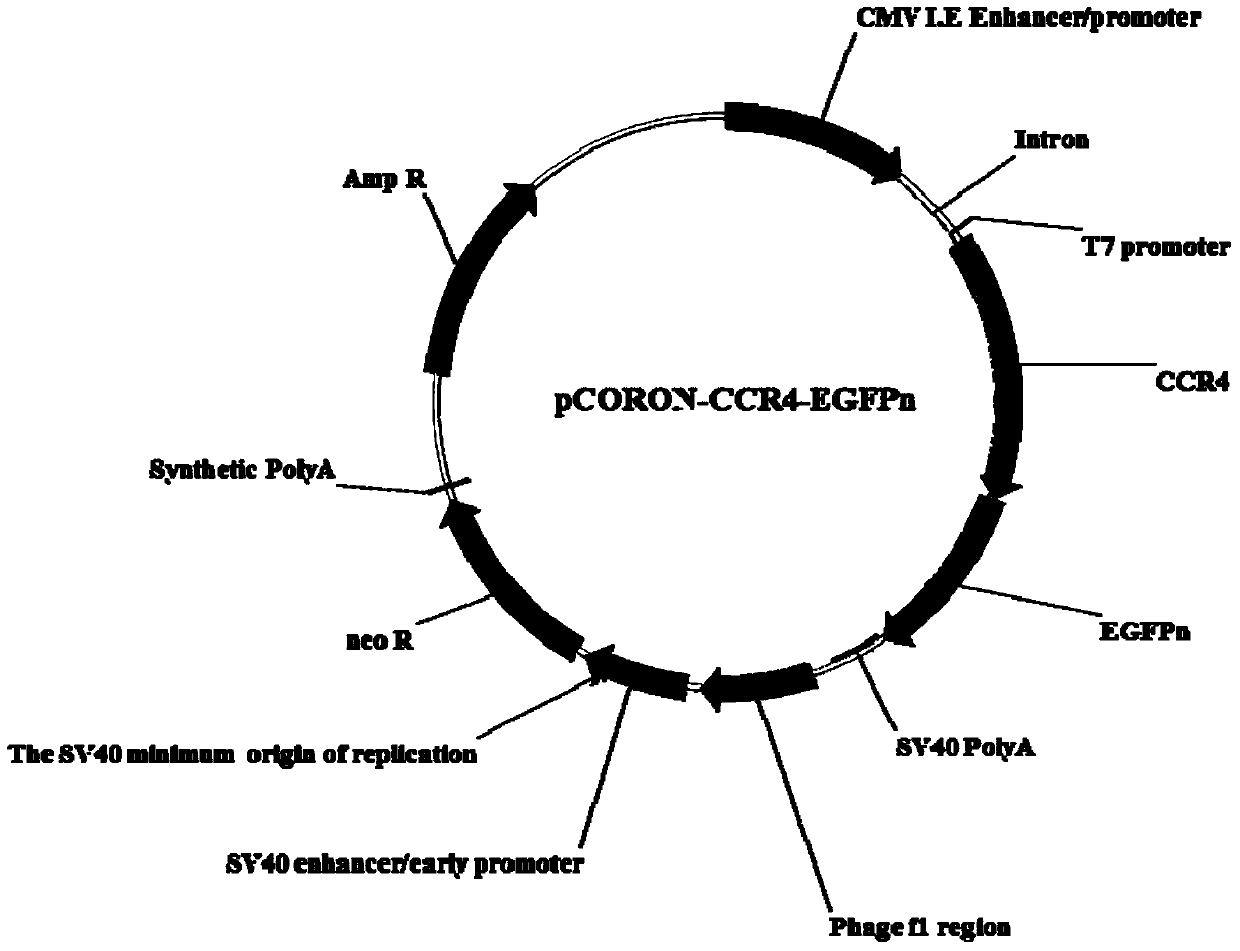
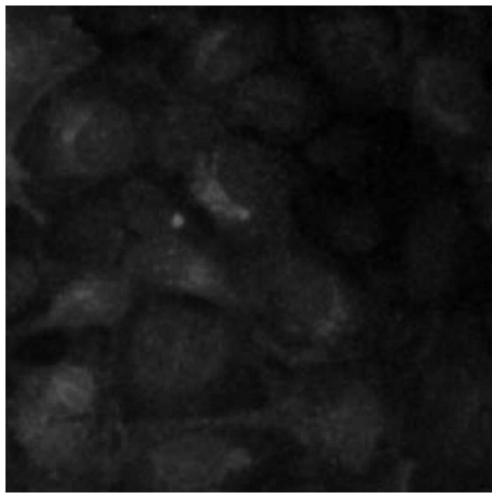
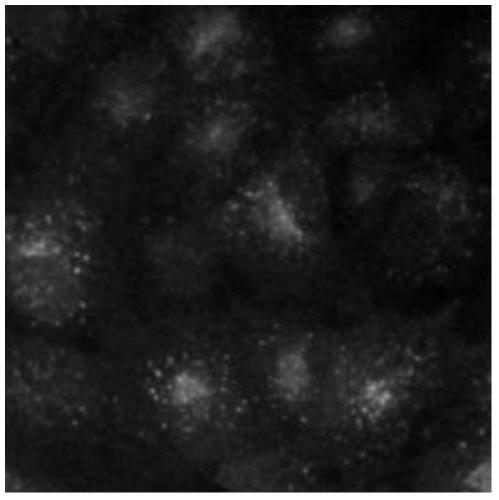
Cell model for screening CCR4 antagonist, and screening method thereof
ActiveCN105462929AEasy to operateStable growthMicrobiological testing/measurementForeign genetic material cellsScreening methodBiological activation
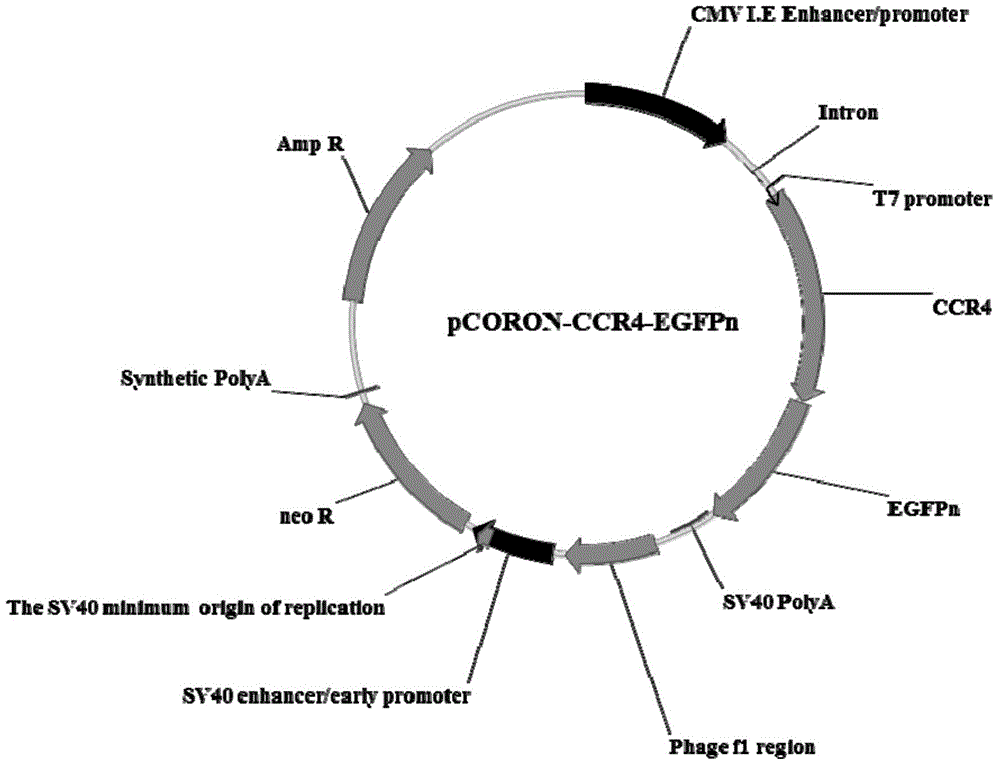
The present invention belongs to the field of pharmacy and cell biology, and relates to an efficient and reliable high content CCR4 antagonist screening cell model using human chemokine receptor 4 as a target, and a screening method thereof. According to the present invention, a cell line being subjected to human CCR4-enhanced green fluorescent protein fusion expression introducing is established, excitation is performed through human thymus activation regulated chemokine and human macrophage chemotatic factor, the CCR4 with green fluorescent protein is induced to produce re-distribution so as to form green fluorescent particles, and the CCR4 antagonist screening cell model for high-content screening is established; the bioactivity of the compound is evaluated by determining the CCR4 green fluorescent particle formation excited or inhibited by the screened compound through the model; and the established drug screening model is the sensitive, efficient and reliable CCR4 antagonist screening cell model suitable for high-throughput and high-content screening, and the method is the sensitive, efficient and reliable method suitable for high-throughput and high-content screening.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Gene engineering bacteria of high efficiency expression of human alpha 1-thymulin and its construction method and use
InactiveCN1616652AHigh expressionLow costBacteriaPeptide/protein ingredientsEscherichia coliThymulin
The present invention is gene engineering bacteria with high efficiency expression of human alpha 1-thmulin and its construction and application, and belongs to the field of bioengineering technology. The gene engineering bacteria is colibacillus DH5-alpha, BL21(DE3) or BLR(DE3), and carry plasmid containing 1-16 alpha 1-thmulin genes. The plasmid promoter is IPTG induced promoter Lac, Tac of PT7, such as plasmid pET series, pGEX series, pQE series, etc. On DNA level, DNA sequences containing alpha 1-thmulin gene are connected serially to form serial body and constitute one series of expression vectors, which transform colibaccilus to obtain one series of gene engineering bacteria with high efficiency expression of human alpha 1-thmulin. The gene engineering bacteria may be used in preparing human alpha 1-thmulin samples. The present invention has high yield and low cost of human alpha 1-thmulin samples.
Owner:EAST CHINA NORMAL UNIV
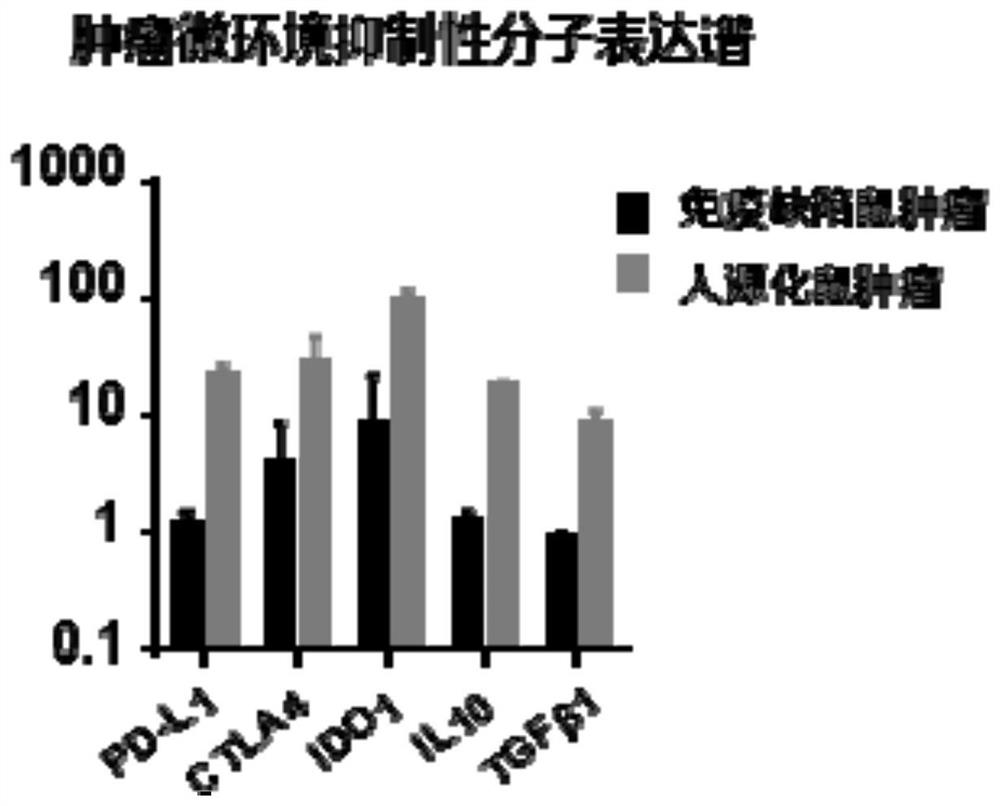
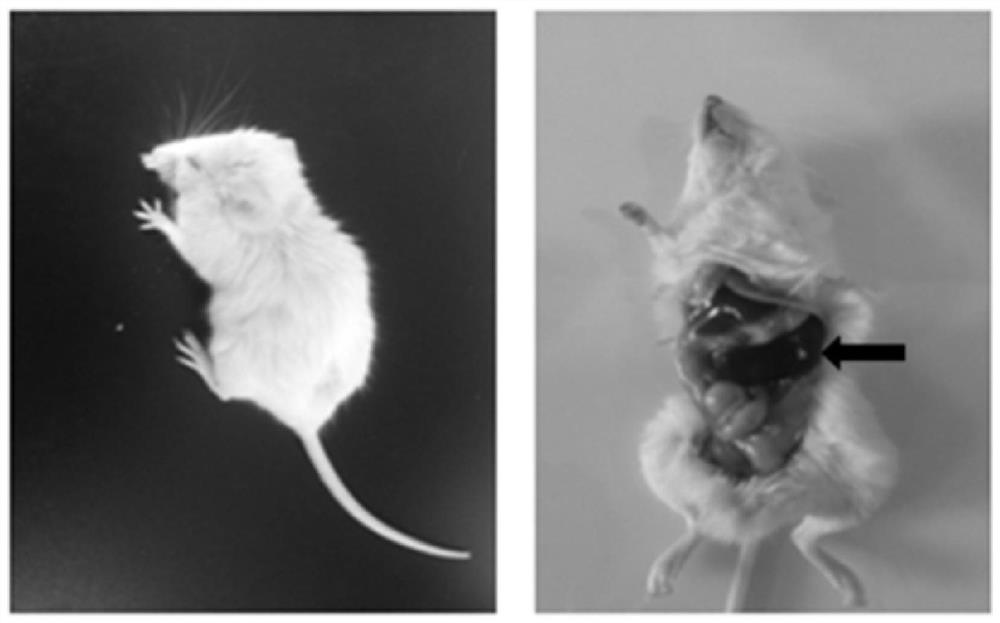
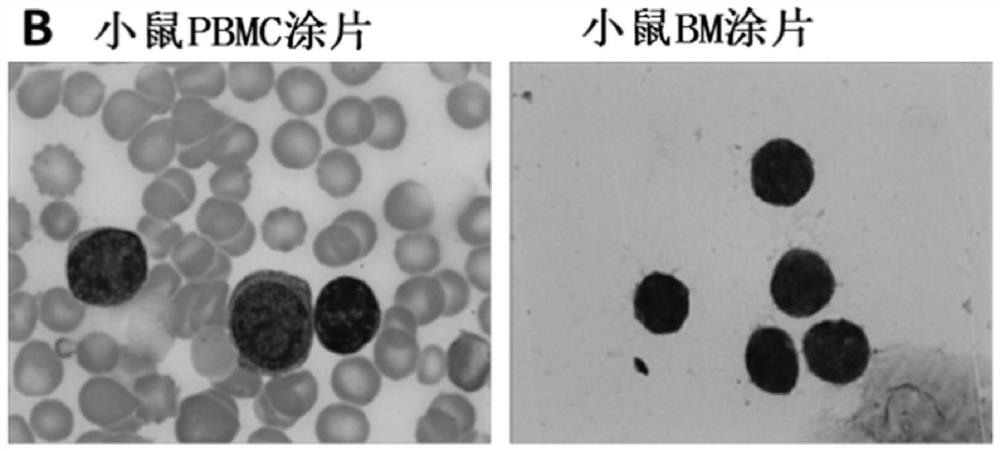
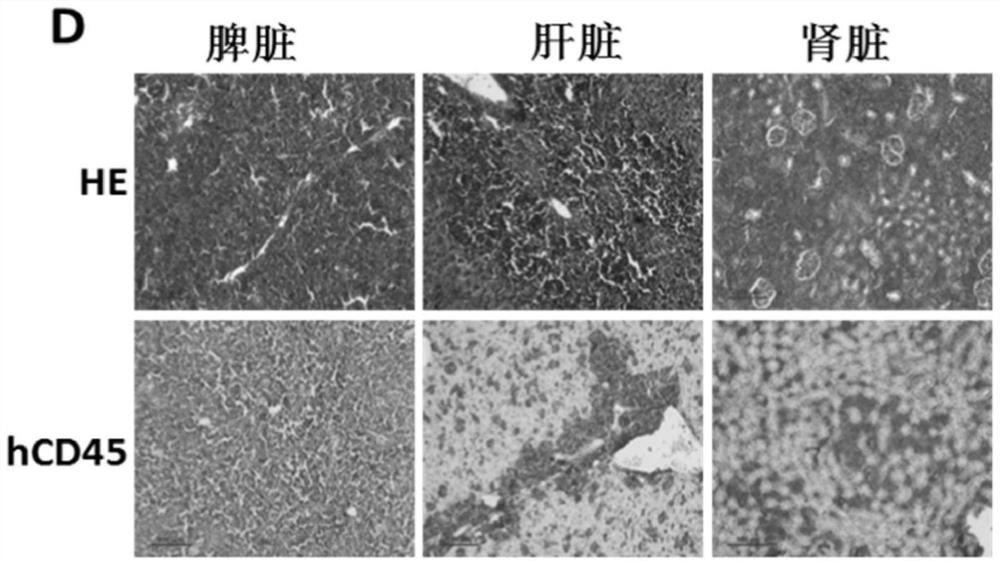
Constructed humanized mouse tumor model and preparation method and application thereof
ActiveCN110934107AMake up for the singularityMake up for completenessAnimal husbandryMouse tumorOncology
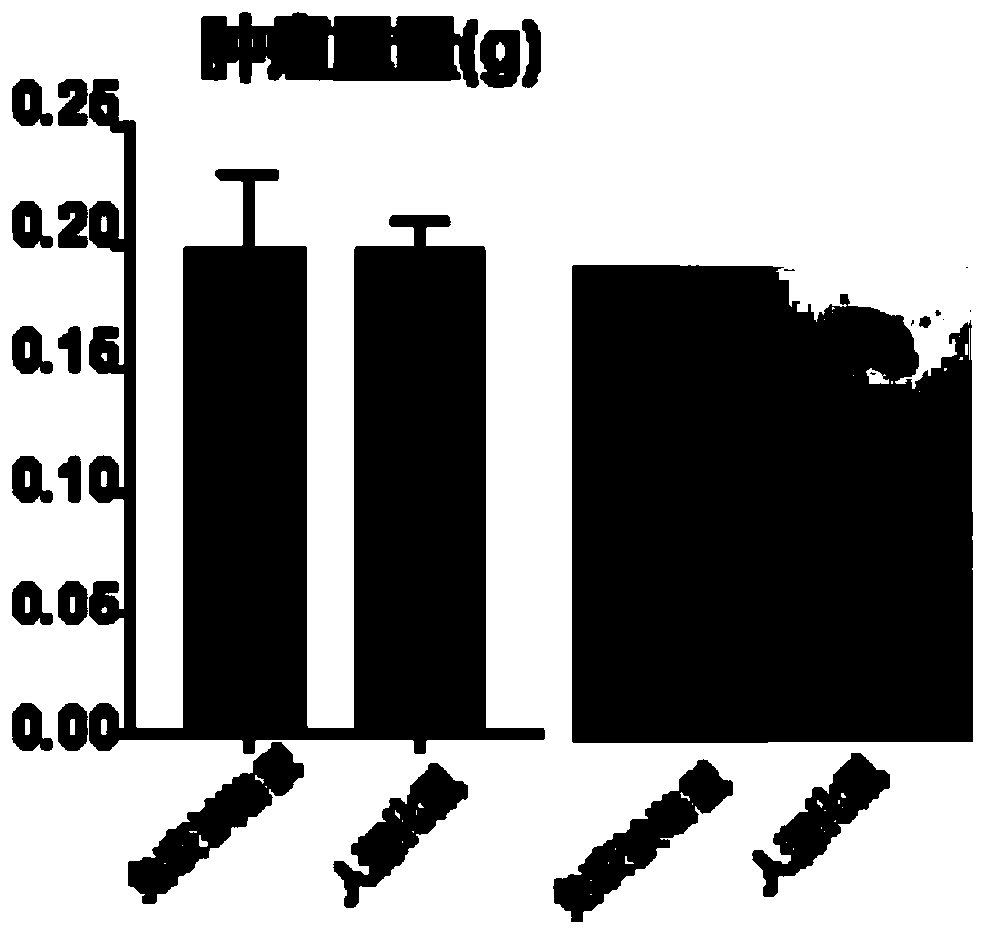
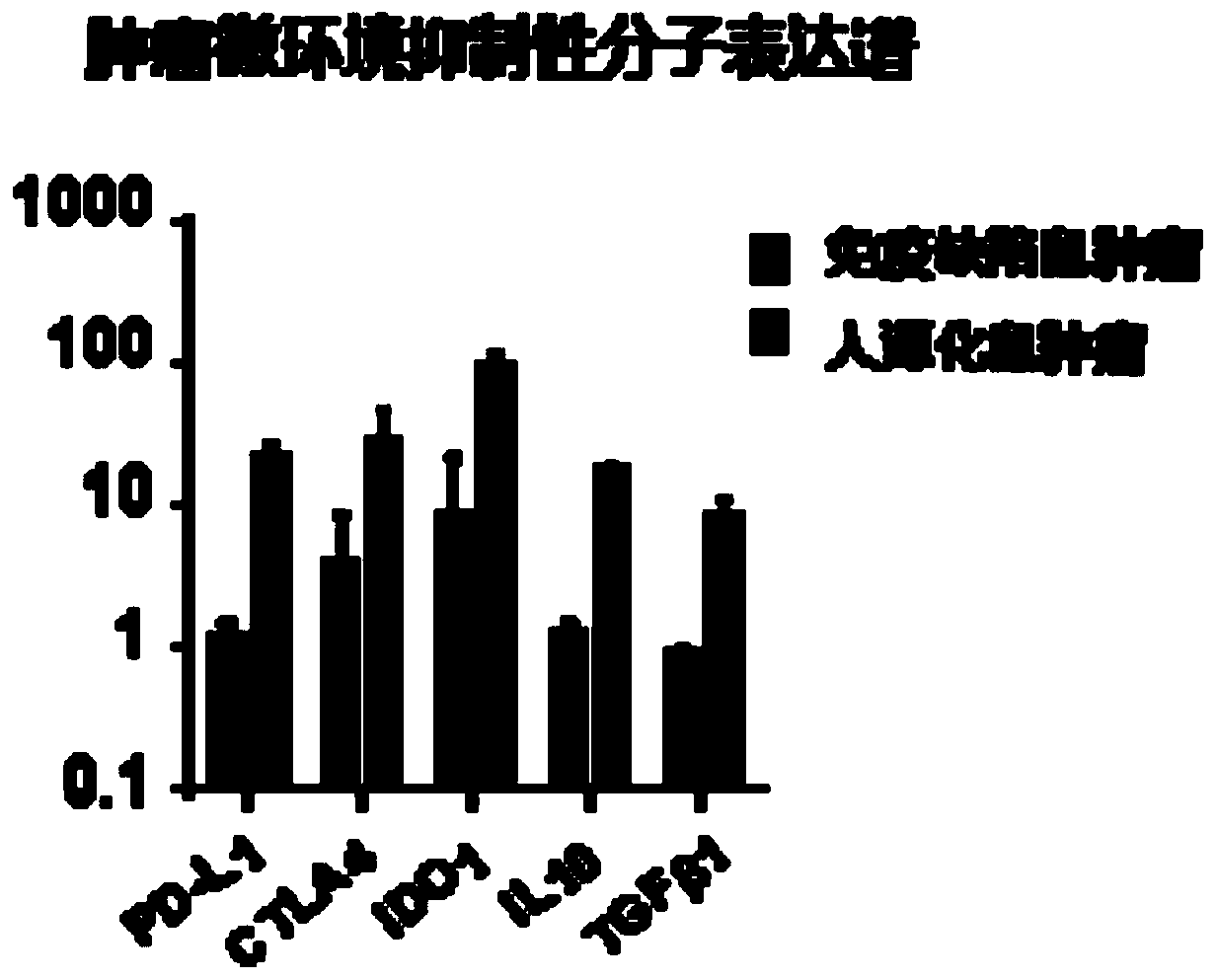
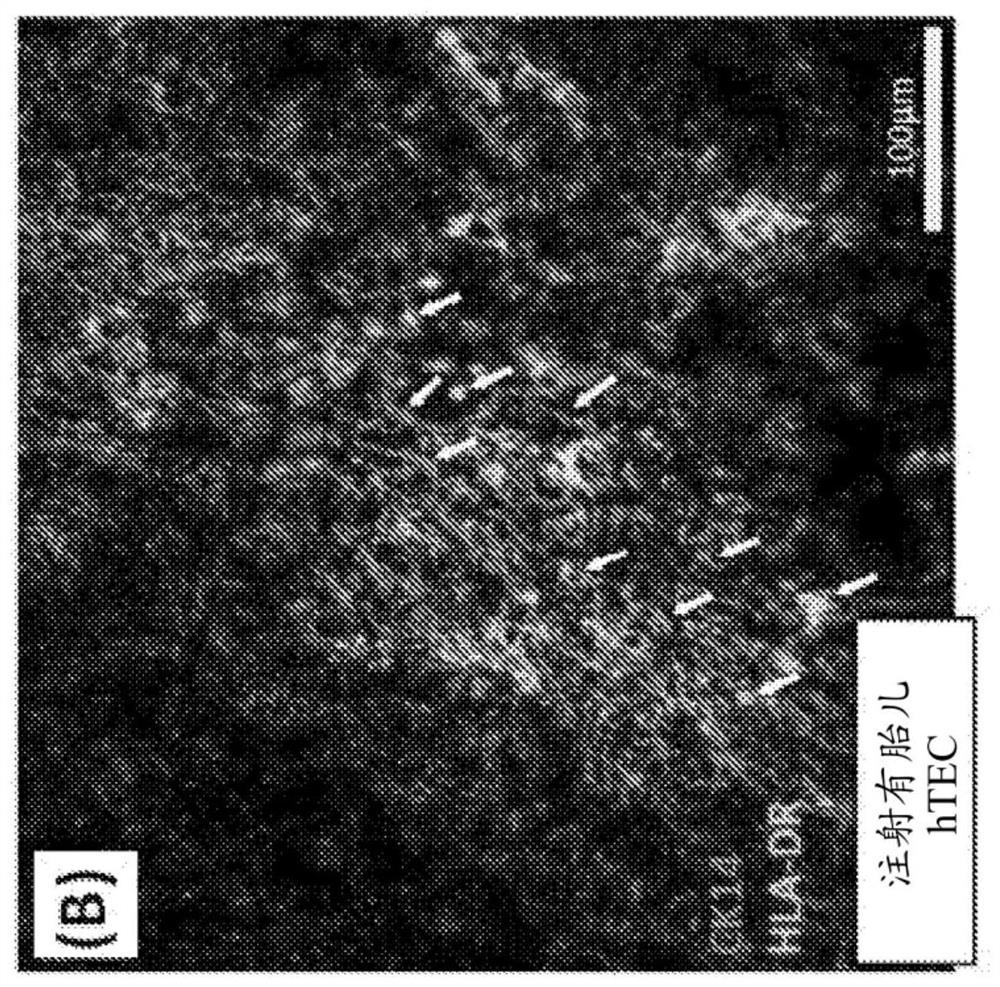
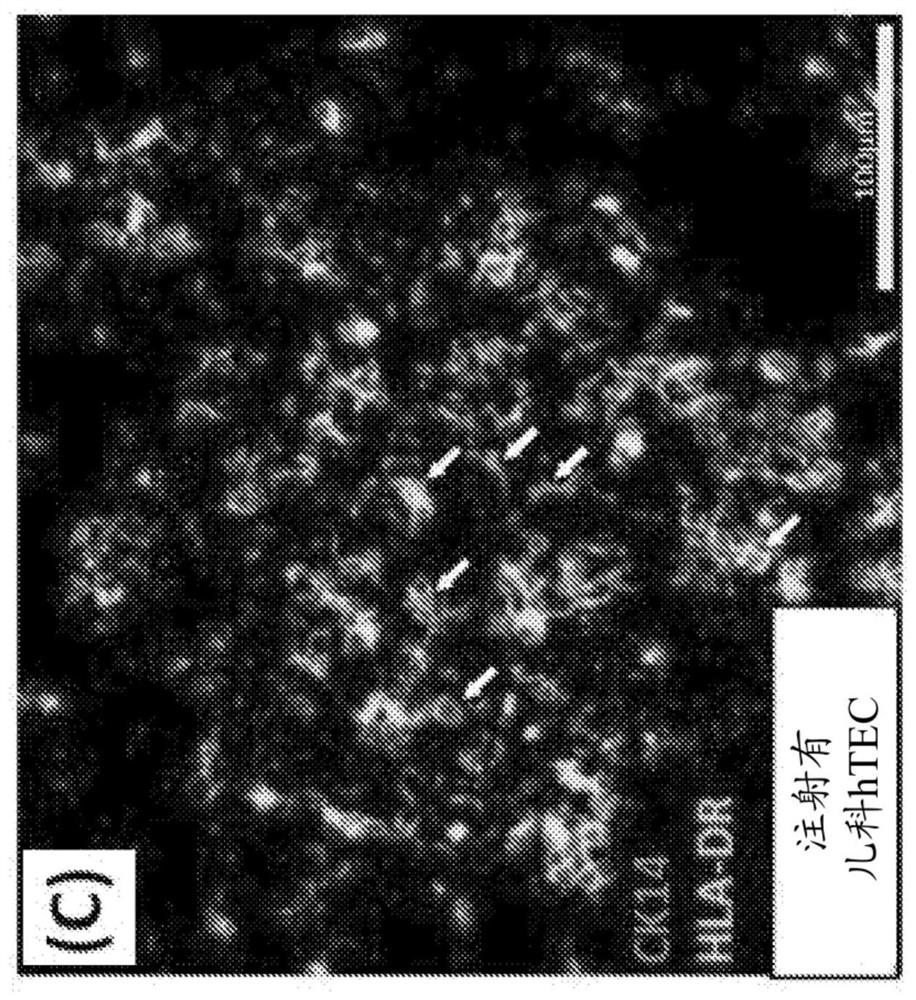
The invention provides a humanized mouse tumor model. The humanized mouse tumor model is characterized by being obtained by transplanting human thymus tissues and CD34+ hematopoietic stem cells homologous with the thymus tissues. The humanized mouse tumor model transplanted by thymus and hematopoietic stem cells is constructed for the first time. The humanized mouse tumor model is determined to have a complete immunosuppression microenvironment, is suitable for overall evaluation of solid tumor immunotherapy, and can be applied to evaluation of feasibility and effectiveness of immunotherapy curative effects. The humanized mouse tumor model makes up for singleness and model incompleteness of existing tumor immunotherapy evaluation methods, and provides reference value for clinical research.Compared with traditional models, the humanized mouse tumor model supplements key effects of a human immune system in immunotherapy.
Owner:中山大学附属第八医院 +1
Peptides for Promoting Hair Growth and Improving Wrinkle and Cosmetic Compositions Comprising the same
ActiveUS20090054349A1Good activity and stabilityThymosin peptidesPeptide/protein ingredientsSkin permeabilityThymosin
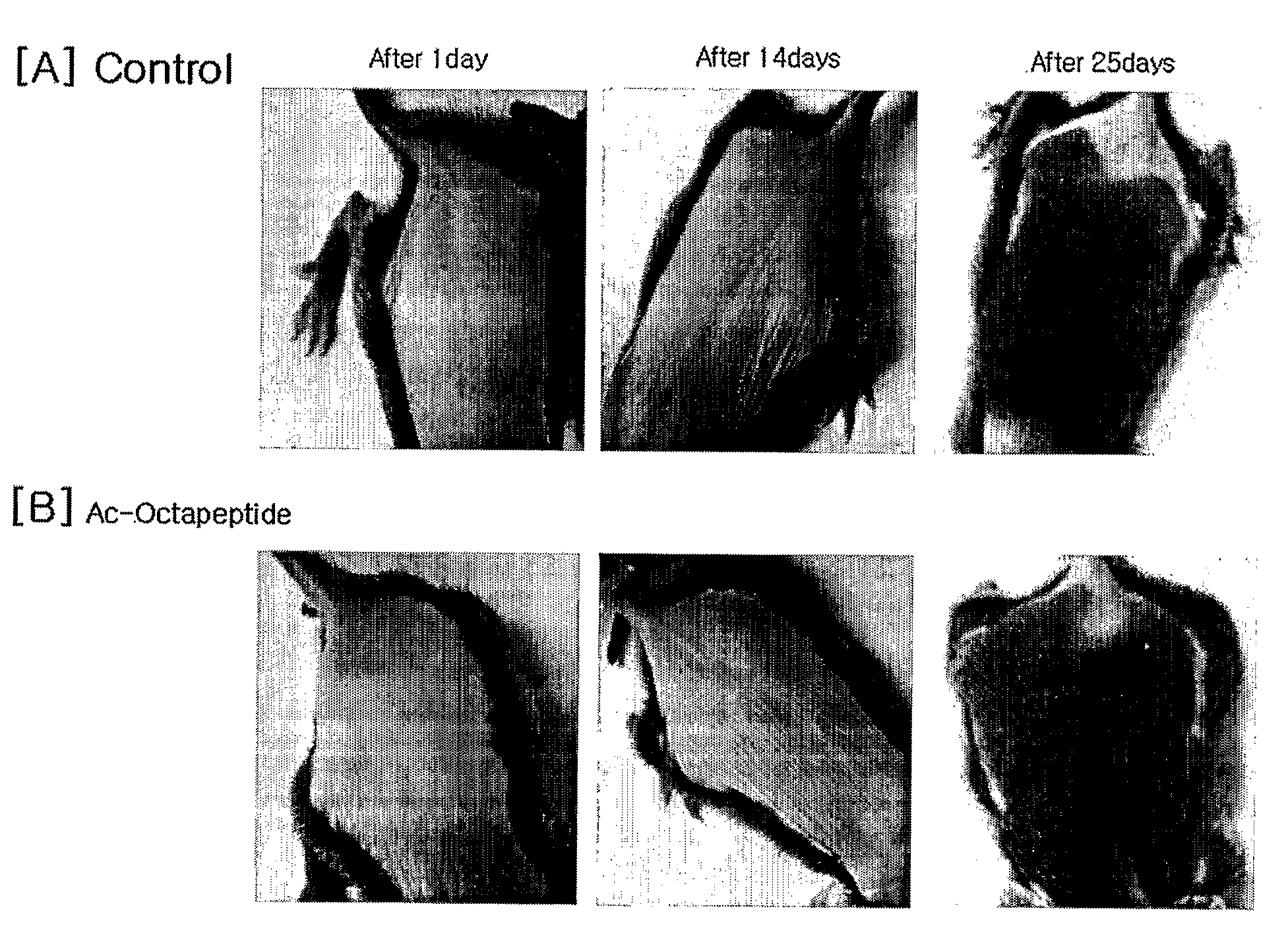
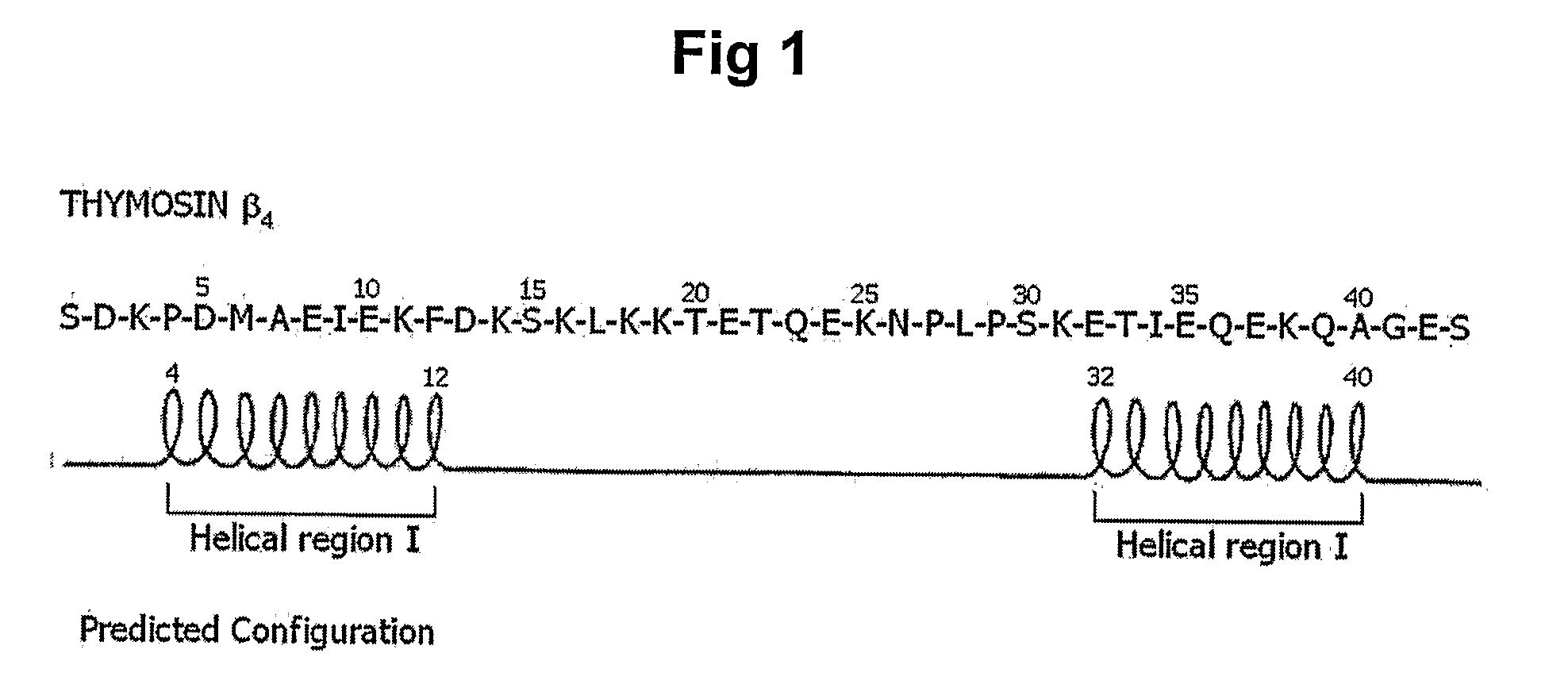
The present invention relates to a peptide comprising a specific amino acid sequence possessing human thymosin β-4 (Tβ4) activities and its uses. The peptide of this invention has identical or similar functions or actions to human Tβ4 and its biological activity is almost identical to natural-occurring Tβ4. In addition, the peptide of this invention exhibits much higher stability and skin permeation than natural-occurring Tβ4. In these connections, the composition comprising the peptides of this invention can exhibit excellent efficacies on improvement in thymosin β-4-effective disorders or conditions. In addition, the peptide of this invention can be advantageously applied to drugs, cosmetics, toothpaste and compositions for mouth cleaning and caring, most preferably, cosmetics. Specifically, the peptide of this invention is advantageously applied to cosmetics for promoting hair growth.
Owner:CAREGEN
Method for recombining, expressing and producing human thymosin in yeast
ActiveCN102925470AEnsure product quality consistencyLow costHybrid peptidesVector-based foreign material introductionNicotiana tabacumRestriction enzyme digestion
The invention provides a method for massively producing human thymosin with low cost. The method is used for implementing restriction enzyme digestion to obtain a fusion gene sequence with a structure as follows: A-X-C, wherein A is a nucleotide sequence of site (1-150)-(1-372) amino acids at the N- end of coded mature human serum albumin; X is the nucleotide sequence of connecting peptide with enterokinase or tobacco etch virus (TEV) protease cutting site contained in a code; and C is a human thymosin gene. The method comprises steps as follows: connecting the fusion gene sequence to an expression carrier; transforming and introducing the expression carrier into saccharomycetes; carrying out induction expression to obtain soluble human serum albumin-thymosin fusion protein; then adding the TEV protease for cutting; and separating and purifying to obtain the recombined human thymosin. The human thymosin production method provided by the invention has the advantages that consistency of quality of products can be ensured, no limitation is generated from sources of raw materials, cost is low, an expression index is high, and mass production can be achieved and the like.
Owner:冯鹏波
Method for producing human thymosin alphal utilizing transgenic tomato
InactiveCN1891824AReduce manufacturing costIncrease contentFermentationVector-based foreign material introductionDiseaseHuman body
The invention relates to a method to produce human thymosin alpha 1 that uses transgene tomato as bio-reactor. It optimizes the nucleotide sequence of human thymosin alpha 1 according to plant preference codon. Compounding the gene and making it connect end to end to form tetrad, then, the plant expression transforming tomato expressed by thymosin alpha 1 cascading gene that is driven by cauliflower 35s promoter would be constructed. The thymosin alpha 1 would have important effect in vivo that could promote T cell function and the producing of special antibody and cell element. It could cure autoimmune diseases like lupus erythematosus, chronicity hepatitis B, etc. It could be also used as assistant medicine for curing cancer and tumor. The fruit containing thymosin alpha 1 could enhance human body immunity.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI +1
Hybrid thymus, methods of making and methods of use for inducing xenograft tolerance, restoring immunocompetence and thymus function
The present disclosure relates to making a hybrid pig-human thymic tissue and using the hybrid thymic tissue to induce tolerance in xenotransplantation. The hybrid thymic tissue can also be used for restoring or inducing immunocompetence in a recipient as well as restoring or promoting the thymus-dependent ability for T cell progenitors to develop into mature functional T cells in a recipient.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Liquid preparation comprising anti-human thymic stromal lymphopoietin (TSLP) monoclonal antibody
ActiveCN114028562ASignificant clinical effectImmunoglobulins against animals/humansPharmaceutical delivery mechanismComplementarity determining regionAntiendomysial antibodies
The invention discloses a liquid preparation of an anti-human thymic stromal lymphopoietin (TSLP) monoclonal antibody. The liquid preparation comprises an anti-human thymic stromal lymphopoietin (TSLP) monoclonal antibody and an amino acid protection agent, wherein the protein concentration of the anti-human thymic stromal lymphopoietin (TSLP) monoclonal antibody is 100-300mg / ml, the amino acid concentration of the amino acid protection agent is 10-500mM, the monoclonal antibody comprises three heavy chain complementarity determining regions (CDR-H1, CDR-H2 and CDR-H3) and three light chain complementarity determining regions (CDR-L1, CDR-L2 and CDR-L3), the amino acid sequence of the CDR-H1 is as shown in SEQ ID NO: 1, the amino acid sequence of the CDR-H2 is as shown in SEQ ID NO: 2, the amino acid sequence of the CDR-H3 is as shown in SEQ ID NO: 3, the amino acid sequence of the CDR-L1 is as shown in SEQ ID NO: 4, the amino acid sequence of the CDR-L2 is as shown in SEQ ID NO: 5, and the amino acid sequence of the CDR-L3 is as shown in SEQ ID NO: 6. The liquid preparation disclosed by the invention is relatively low in viscosity and can be easily injected by a syringe, so that the liquid preparation can be used as an injection, especially a hypodermic injection.
Owner:QYUNS THERAPEUTICS CO LTD
A kind of cell model and screening method for screening CCR4 antagonists
ActiveCN105462929BEasy to operateStable growthMicrobiological testing/measurementForeign genetic material cellsScreening methodBiological activation
The present invention belongs to the field of pharmacy and cell biology, and relates to an efficient and reliable high content CCR4 antagonist screening cell model using human chemokine receptor 4 as a target, and a screening method thereof. According to the present invention, a cell line being subjected to human CCR4-enhanced green fluorescent protein fusion expression introducing is established, excitation is performed through human thymus activation regulated chemokine and human macrophage chemotatic factor, the CCR4 with green fluorescent protein is induced to produce re-distribution so as to form green fluorescent particles, and the CCR4 antagonist screening cell model for high-content screening is established; the bioactivity of the compound is evaluated by determining the CCR4 green fluorescent particle formation excited or inhibited by the screened compound through the model; and the established drug screening model is the sensitive, efficient and reliable CCR4 antagonist screening cell model suitable for high-throughput and high-content screening, and the method is the sensitive, efficient and reliable method suitable for high-throughput and high-content screening.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
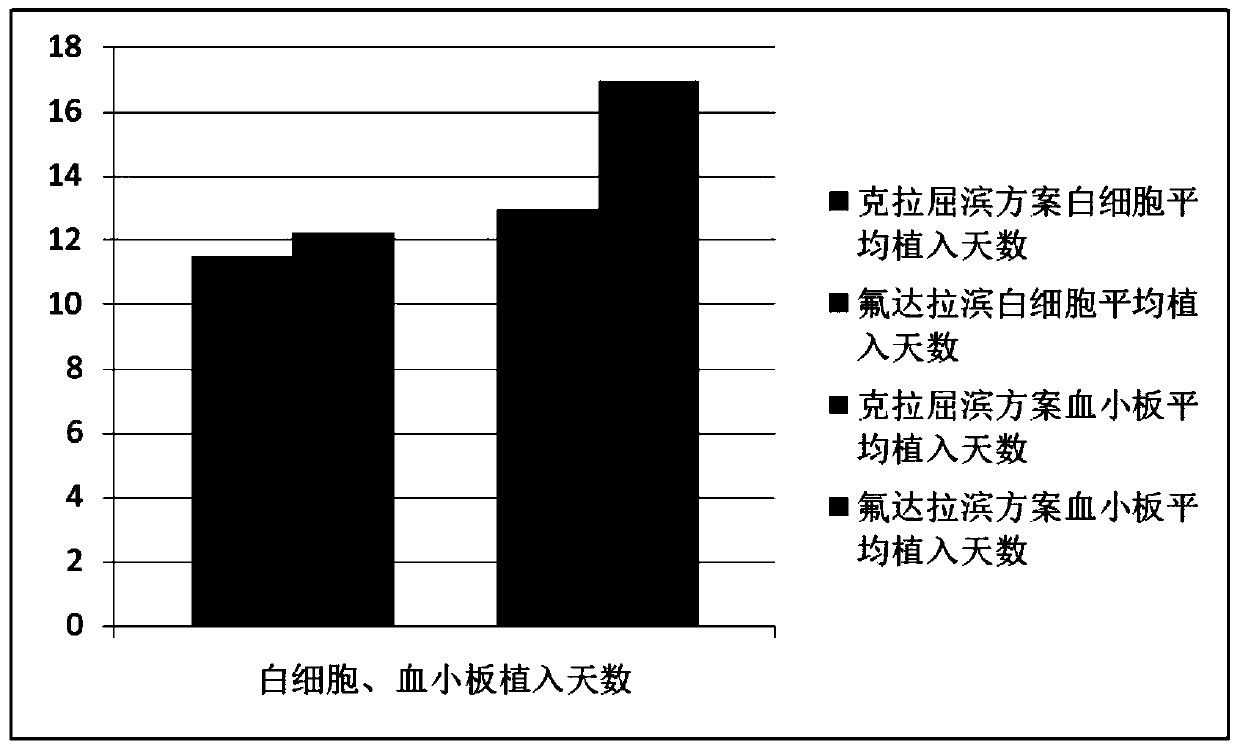
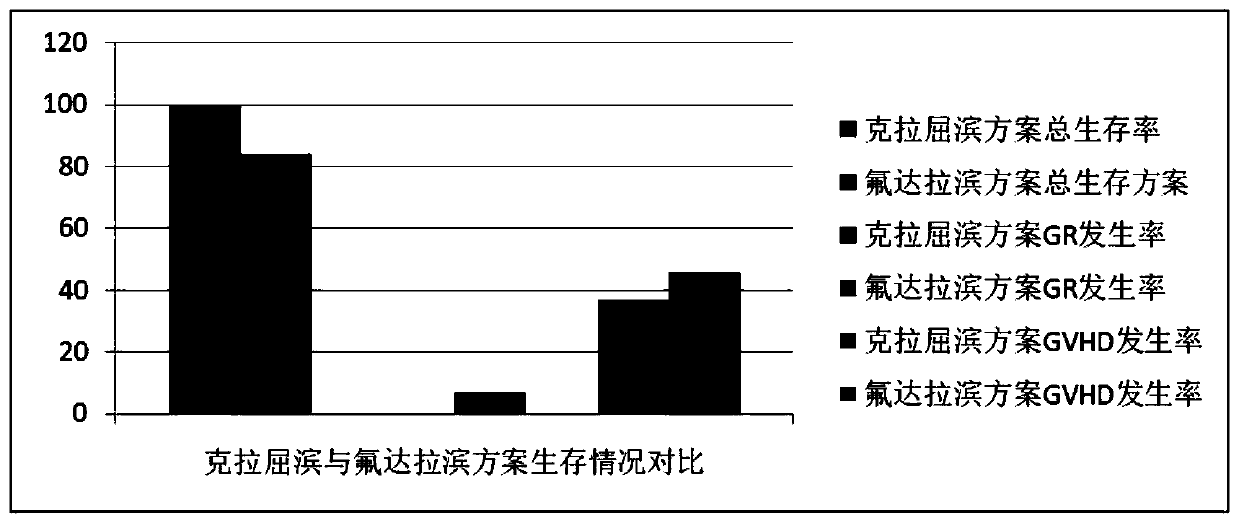
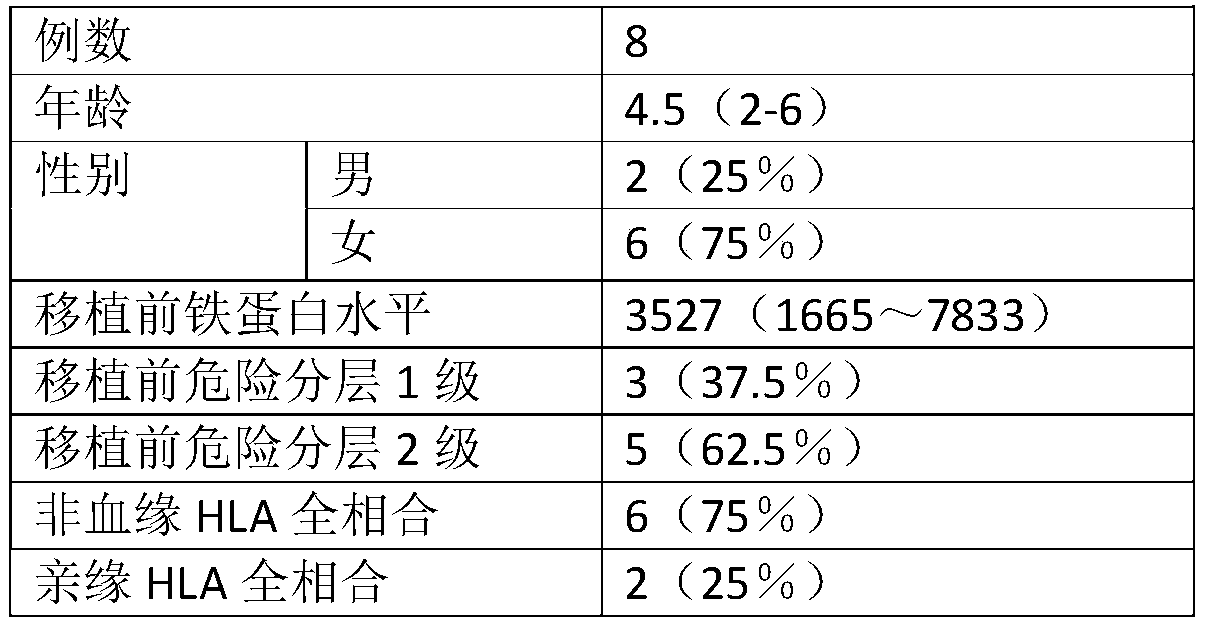
Combined chemotherapeutic agent for transplantation pretreatment of thalassemia stem cells in children
InactiveCN109771643AImprove complianceImprove toleranceOrganic active ingredientsAntibody ingredientsLarge doseThalassemia
The invention discloses a combined chemotherapeutic agent for transplantation pretreatment of thalassemia stem cells in children, which comprises the following components: 5 mg / m<2>*5 days of cladrobin, 1.8 g / m<2>*2 days of cyclophosphamide, 4-4.8 mg / kg*3 days of busulfan and 2.5 mg / kg*2 days of anti-human thymus globulin. The combined chemotherapeutic agent is based on the cladrobin, and the cladrobin is combined with the cyclophosphamide, the busulfan and anti-human thymus globulin to perform pre-treatment large-dose chemotherapy before transplantation of Mediterranean stem cells of children, so that the compliance and tolerance of the chemotherapy are improved, the immunosuppression strength is increased, the transplantation success rate of transplantation of stem cells of a patient isimproved, the transplantation-related complications are reduced, the transplantation curative effect is improved, the survival time of the patient is prolonged, and the combined chemotherapeutic agenthas good social benefit and economic benefit and wide clinical application prospect. The efficient and safe pretreatment scheme can be used for transplantation of thalassemia stem cells in children,and the transplantation survival rate is improved.
Owner:ZHONGSHAN HOSPITAL XIAMEN UNIV
A kind of anti-human tslp monoclonal antibody and application thereof
ActiveCN113683694BGood neutralizing activityQuite affinitySenses disorderDigestive systemDiseaseComplementarity determining region
This application provides an anti-human thymus stromal lymphopoietin (TSLP) monoclonal antibody and its application. The anti-human thymus stromal lymphopoietin (TSLP) monoclonal antibody of the present application comprises three heavy chain complementarity determining regions (CDR‑H1, CDR‑H2 and CDR‑H3) and three light chain complementarity determining regions (CDR‑L1 , CDR‑L2 and CDR‑L3), wherein, (a) the amino acid sequence of CDR‑H1 is shown in SEQ ID NO: 1; (b) the amino acid sequence of CDR‑H2 is shown in SEQ ID NO: 2; (c ) The amino acid sequence of CDR‑H3 is shown in SEQ ID NO: 3; (d) the amino acid sequence of CDR‑L1 is shown in SEQ ID NO: 4; (e) the amino acid sequence of CDR‑L2 is shown in SEQ ID NO: 5 and (f) the amino acid sequence of CDR-L3 is shown in SEQ ID NO:6. The anti-human thymus stromal lymphopoietin (TSLP) monoclonal antibody, compared with the anti-human TSLP monoclonal antibody Tezepelumab (expressed and prepared according to the sequence disclosed in the patent), has comparable affinity for binding human TSLP, and is in the cell level. It is more active than Tezepelumab and is expected to show good clinical effects in the prevention and treatment of related diseases.
Owner:QYUNS THERAPEUTICS CO LTD
A kind of construction humanized mouse tumor model and its preparation method and application
The invention provides a humanized mouse tumor model, which is characterized in that the humanized mouse tumor model is obtained by transplanting human thymus tissue and CD34+ hematopoietic stem cells homologous to the thymus tissue. The present invention constructs a humanized mouse tumor model transplanted from thymus and hematopoietic stem cells for the first time. The humanized mouse tumor model has confirmed that it has a complete immunosuppressive microenvironment, and is suitable for the overall evaluation of solid tumor immunotherapy, and can be used to evaluate the feasibility and effectiveness of immunotherapy. The invention makes up for the singleness and incompleteness of the existing tumor immunotherapy evaluation methods, provides reference value for clinical research, and complements the key role of the human immune system in immunotherapy compared with traditional models.
Owner:THE EIGHTH AFFILIATED HOSPITAL SUN YAT SEN UNIV +1
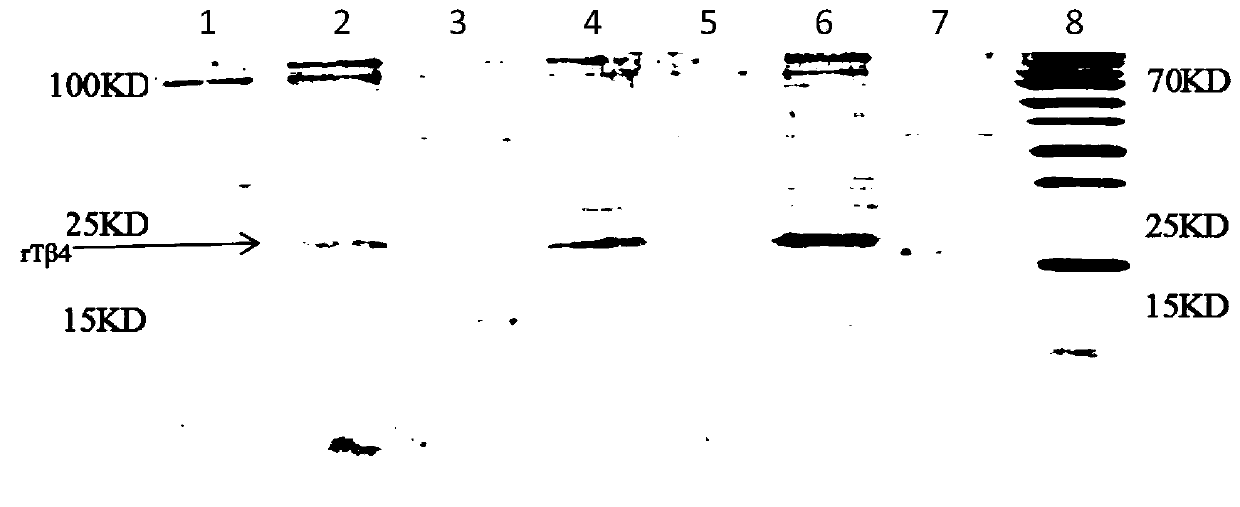
Polyethylene glycol modified human thymosin beta 4 tandem repeat protein, as well as preparation method and application thereof
InactiveCN110964117AEasy to operateLow experimental requirementsThymosin peptidesPeptide/protein ingredientsProtein targetTandem repeat
The invention discloses a polyethylene glycol modified human thymosin beta 4 tandem repeat protein (PEG-rTbeta4), as well as a preparation method and application thereof. The PEG-rTbeta4 is obtained after recombinant human thymosin beta 4 tandem repeat protein is subjected to specific chemical modification with polyethylene glycol, wherein the rTbeta4 has gene sequence codes connected in the following sequence: a HKCDI gene sequence, a thymosin beta4 gene sequence, a GS Linker GSGSG-thymosin beta4 gene sequence and a 6*His label gene sequence. Furthermore, the invention discloses a method forpreparing the protein. The preparation method can be used for preparing target protein having the purity of 90 percent or more, has the advantages of low cost, high bioactivity and the like, and is simple and quick. Experiments prove that the PEG-rTbeta4 protein has the effects of promoting proliferation and migration of myocardial cells, resisting apoptosis, promoting hair growth and angiogenesisand accelerating wound healing. Therefore, the PEG-rTbeta4 protein has a wide application prospect in heart function recovery after myocardial infarction, hair growth promotion and wound healing acceleration.
Owner:HARBIN MEDICAL UNIVERSITY
A method for establishing human hematological tumor pdx model
ActiveCN109481666BHigh xenograft survival rateHigh tumor formation rateMammal material medical ingredientsAntibody ingredientsHuman leukemiaIntravenous gammaglobulin
The invention discloses a method for establishing a PDX (Patient Derived Xenograft) model of human blood tumor. The method comprises the steps of extracting a blood tumor cell of a patient, adding rabbit anti-human thymocyte immunoglobulin ATG (Anti-Thymocyte Globulin) and patient autologous serum, mixing, incubating and after completing the incubation, re-suspending the obtained cell and inoculating in a mouse; and feeding the inoculated mouse with CsA (Cyclosporine A) and lasting for 5-10 days starting from 2 days before the inoculation of the blood tumor cell. The method disclosed by the invention has the beneficial effects that a novel highly immunodeficient NCG mouse independently developed in China is firstly adopted to establish a human leukemia PDX model; by orally taking human thymocyte immunoglobulin pretreatment sample combined with the cyclosporine for a long term, donor-derived T cells are removed and inhibited; and by combining the toxic effect of the ATG on lymphocytes with the functional blocking effect of the CsA on T lymphocytes, the immune function of the T lymphocytes can be persistently inhibited and the success rate of PDX modeling of the blood tumor is significantly improved.
Owner:JIANGSU PROVINCIAL HOSPITAL OF TCM
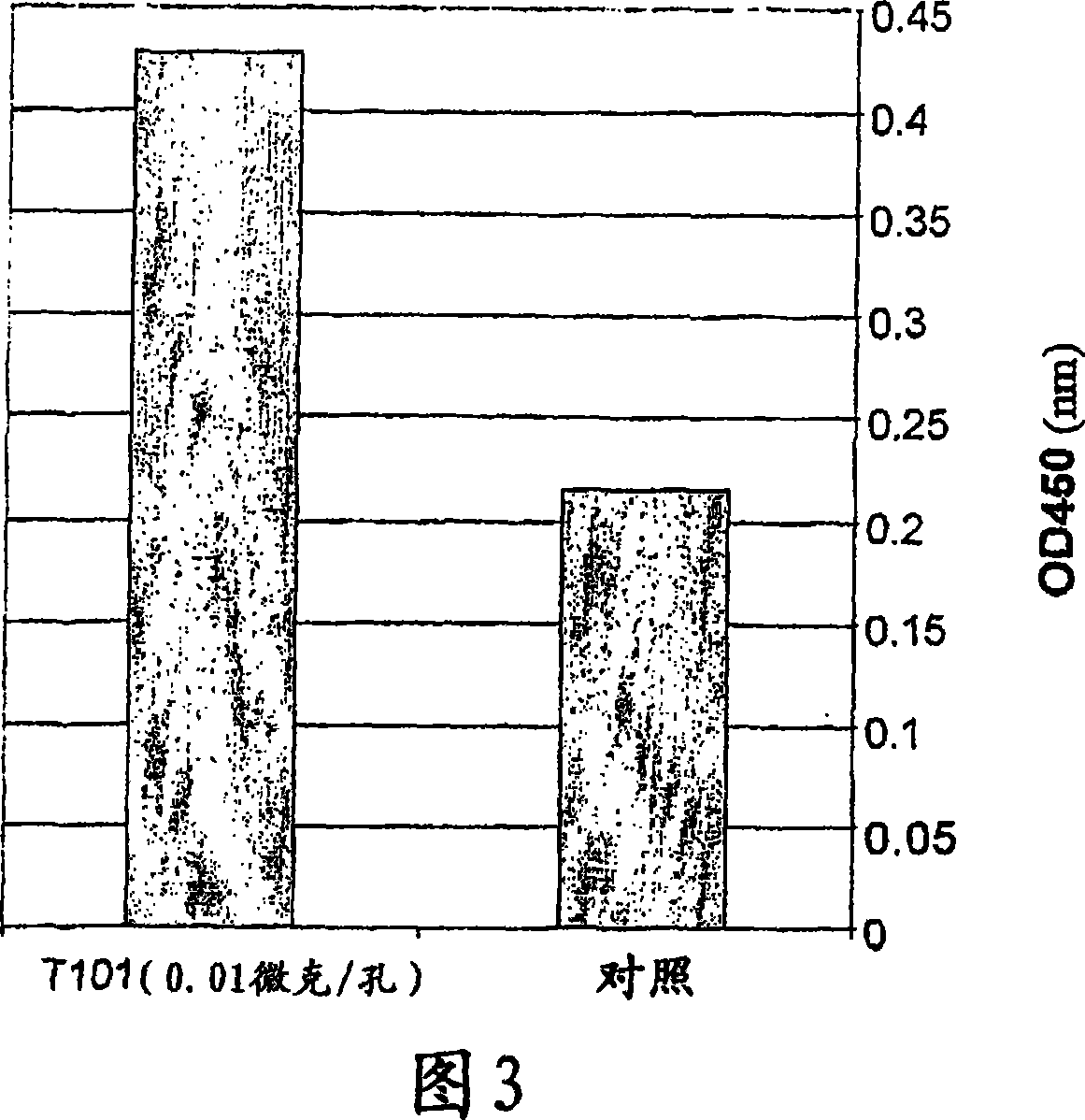
A thymus-specific protein
The invention provides the novel thymus-specific human protein T101, an 84-amino acid polypeptide isolated from the human thymus. The full T101 peptide contains a 33-amino acid signal peptide and a 51-amino acid T101 peptide sequence with both immune stimulatory and inhibitory activities. Also provided are modified peptides and partial T101 peptide sequences.
Owner:IMMUNE SYST KEY
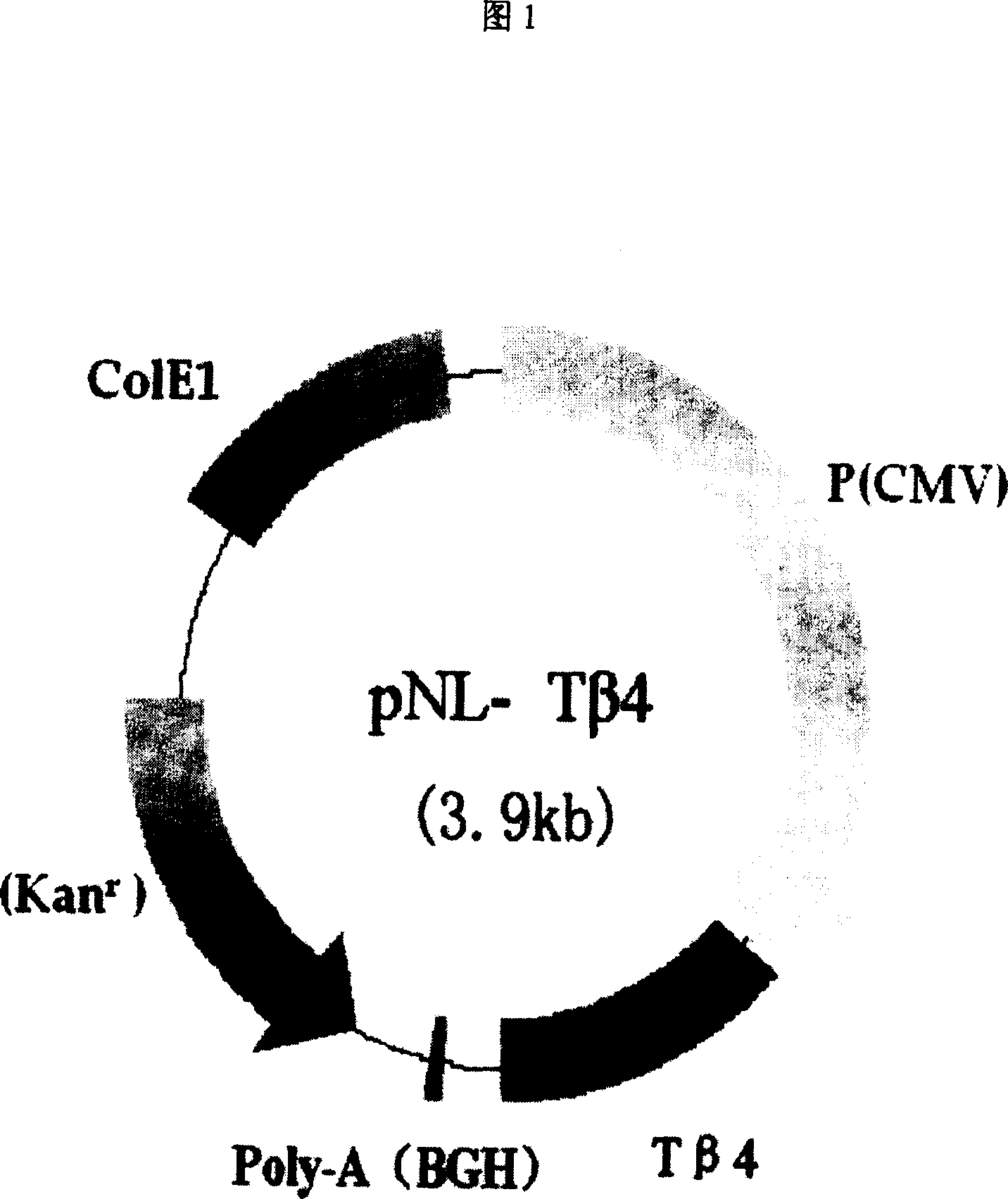
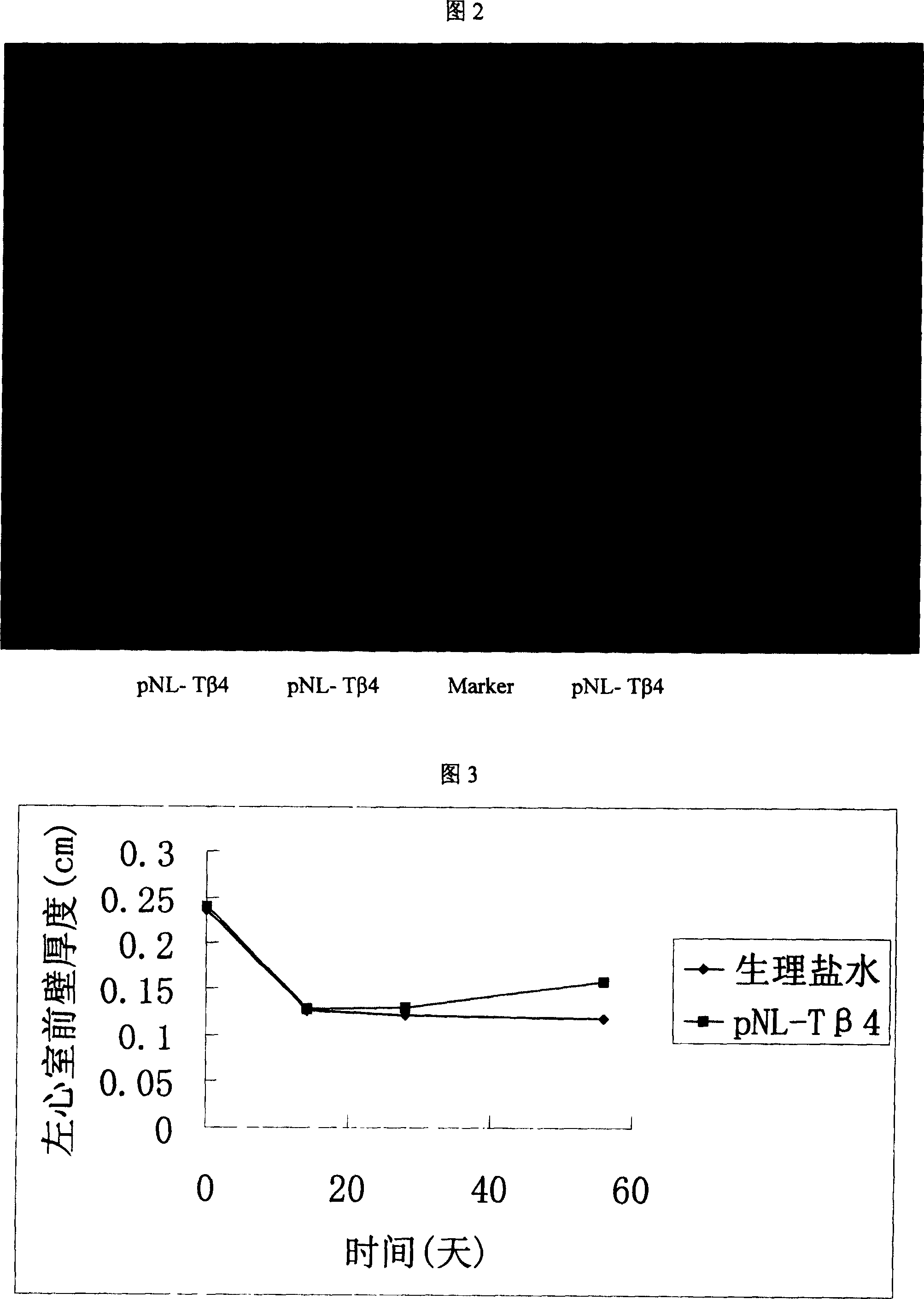
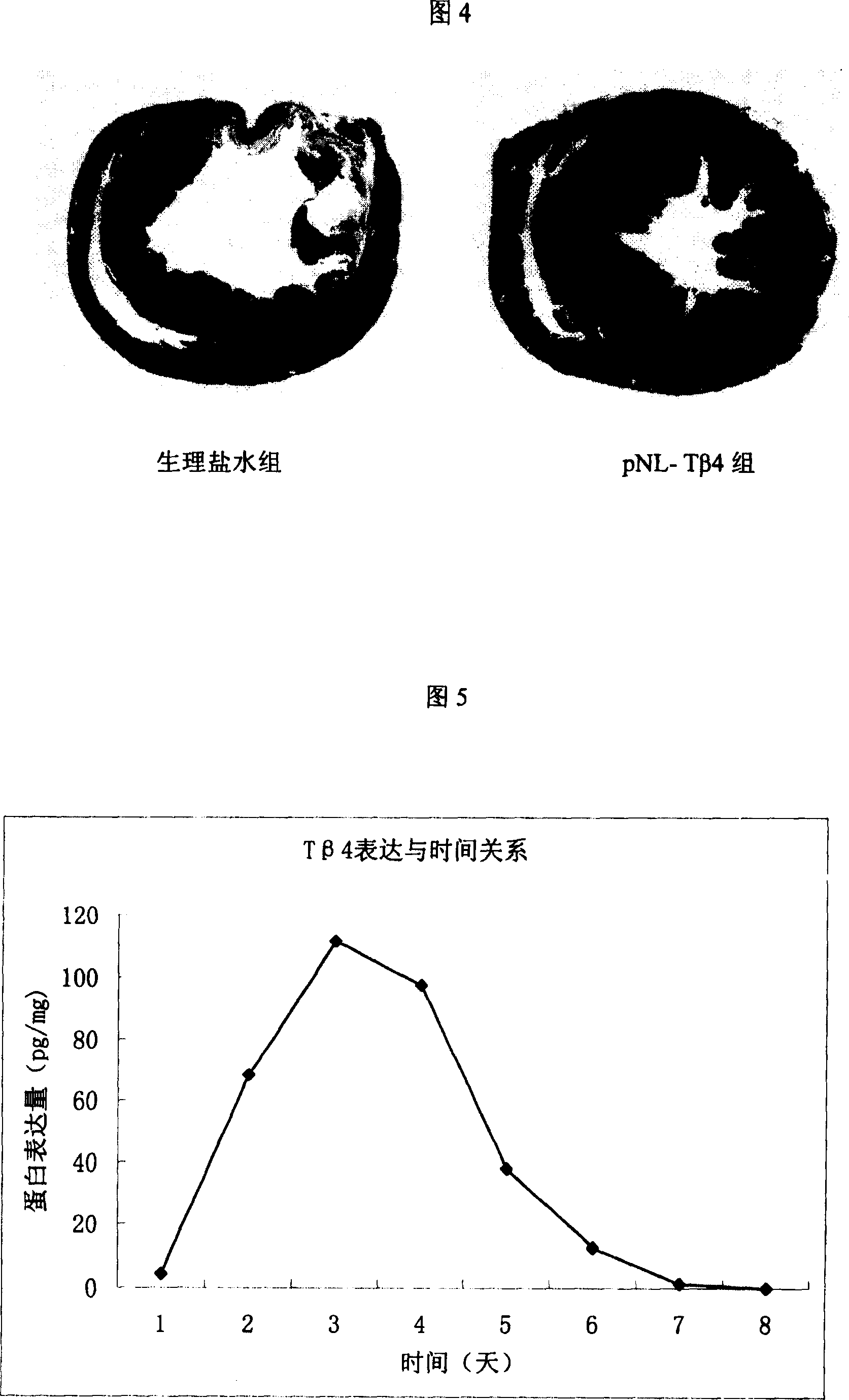
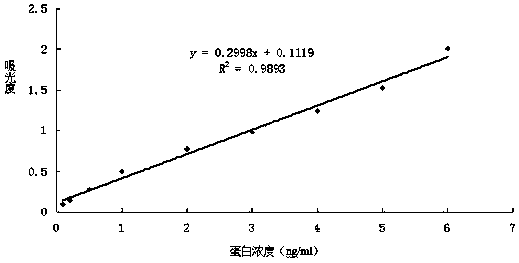
Recombinant plasmid with human thymosin Beta-4gene
ActiveCN100372572CPromote migrationHigh activityHormone peptidesPeptide/protein ingredientsPromoterGene carrier
Owner:BEIJING NORTHLAND BIOTECH
Method of preparing natural human thymosin alpha 1 using series expression mode
ActiveCN100379863CSimple processSimple and fast operationFermentationVector-based foreign material introductionEscherichia coliPolynucleotide
The invention discloses a method to manufacture biology polypeptide, especially a method to make natural human thymosin by gene series express method. It contains compounding two polynucleotide section, compounding double enzyme cut, T alpha 1 gene three times connecting in series, constructing high efficiency expression, constructing engineering fungus, expressing T alpha 1 polypeptide six series bodies in engineering fungus, purifying and cracking. The invention could abundantly express aim albumen, and it has simple technology, easy to operate and low cost.
Owner:广东暨大基因药物工程研究中心有限公司
In-vitro expression and separation and purification method for chemotactic factor TARC/CCL17
InactiveCN108329389ALow costReduce the difficulty of productionChemokinesPeptide preparation methodsEscherichia coliPurification methods
The invention discloses a method of in-vitro recombinant expression of human thymus activation regulated chemokine TARC / CCL17. In the method, human TARC / CCL17 protein is obtained through fusion expression of escherichia coli; then by means of protease digestion, affinity chromatography, gel chromatography and ion-exchange column chromatography, the expression product is purified to obtain pure human TARC / CCL17 protein. The method achieves massive soluble expression of the human TARC / CCL17 protein, which can be used as an ELISA standard substance. The method greatly reduces production cost anddifficulty of the TARC / CCL17 protein.
Owner:SHENZHEN PKU HKUST MEDICAL CENT
Human thymosin β4 triploid protein, coding gene and separation and purification method thereof
InactiveCN103864916BEasy to separate and purifyHigh yieldThymosin peptidesPeptide preparation methodsLymphocyteHair removers
The present invention relates to human thymosin β4 triploid protein, encoding genes and methods for isolating and purifying the same. The amino acid sequence of the human thymosin β4 triploid protein involved is shown in SED ID NO: 1; the coding gene sequence involved is shown in SED ID NO: 2; the separation and purification method involved is to use an affinity chromatography column Carry out separation and purification. The recombinant human thymosin β4 tandem triploid of the present invention can significantly improve the immune response performance of T-lymphocytes as detected by the rosette experiment; the mouse fur growth experiment shows that the recombinant human thymosin β4 tandem triploid can significantly promote hair removal agent treatment in mice Growth in the fur.
Owner:NORTHWEST UNIV
Human Thymic Stromal Lymphopoietin Monoclonal Antibody and Its Application
ActiveCN111196850BHigh affinityInhibition of activationAntipyreticAnalgesicsAllergic dermatitisAntiendomysial antibodies
The invention relates to the technical field of antibodies, in particular to a human thymus stromal lymphopoietin monoclonal antibody and an application thereof. The TSLP antibody provided by the present invention can specifically bind hTSLP with high affinity, and can effectively inhibit the binding of TSLP to its receptor, thereby inhibiting TSLP from activating downstream signaling pathways and activating immune cells. The TSLP antibody provided by the present invention is used for the content detection of hTSLP and allergic asthma, chronic obstructive pulmonary disease, allergic dermatitis, allergic rhinitis, allergic sinusitis, eosinophilic esophagitis, allergic conjunctivitis, inflammatory bowel disease It is of great significance in the diagnosis, treatment and prognosis of inflammatory or allergic inflammatory diseases such as atopic dermatitis or atopic dermatitis.
Owner:IPHASE THERAPEUTICS LTD
A kind of preparation method of anti-human thymus stromal lymphopoietin (tslp) monoclonal antibody concentrated solution
ActiveCN114028561BSignificant clinical effectImmunoglobulins against cytokines/lymphokines/interferonsPharmaceutical delivery mechanismHigh concentrationAntiendomysial antibodies
The application discloses a method for preparing a concentrated solution of anti-human thymus stromal lymphopoietin (TSLP) monoclonal antibody, comprising the following steps: Concentrate the solution to obtain the first concentrated sample; Step 2: Replace the first concentrated sample with a buffer solution to obtain a replacement concentrated solution; Step 3: Mix the replaced concentrated solution and the mother liquor of the amino acid protective agent to obtain a mixed solution, and the The above mixed solution was concentrated by ultrafiltration to obtain a second concentrated sample. The preparation method described in this application can reduce the viscosity of high-concentration antibody drug solution and improve the stability. The method is simple and easy, and can be scaled up for production, which can ensure the high purity of the sample and obtain a high recovery rate.
Owner:QYUNS THERAPEUTICS CO LTD
Liquid formulation comprising anti-human thymic stromal lymphopoietin (tslp) monoclonal antibody
ActiveCN114028562BSignificant clinical effectImmunoglobulins against animals/humansPharmaceutical delivery mechanismComplementarity determining regionAntiendomysial antibodies
This application discloses a liquid preparation of anti-human thymus stromal lymphopoietin (TSLP) monoclonal antibody, comprising anti-human thymus stromal lymphopoietin (TSLP) monoclonal antibody and amino acid protective agent, anti-human thymus stromal lymphocytes The protein concentration of the TSLP monoclonal antibody is 100-300mg / ml, and the amino acid concentration of the amino acid protection agent is 10-500mM. The monoclonal antibody comprises three heavy chain complementarity determining regions (CDR‑H1, CDR‑H2 and CDR‑H3) and three light chain complementarity determining regions (CDR‑L1, CDR‑L2 and CDR‑L3), wherein: The amino acid sequence of CDR‑H1 is shown in SEQ ID NO: 1; the amino acid sequence of CDR‑H2 is shown in SEQ ID NO: 2; the amino acid sequence of CDR‑H3 is shown in SEQ ID NO: 3; the amino acid of CDR‑L1 The sequence is shown in SEQ ID NO: 4; the amino acid sequence of CDR‑L2 is shown in SEQ ID NO: 5; the amino acid sequence of CDR‑L3 is shown in SEQ ID NO: 6. The liquid formulation of the present application has a low viscosity and can be easily injected with a syringe, so it can be used as an injection, especially a subcutaneous injection.
Owner:QYUNS THERAPEUTICS CO LTD
Kit used for diagnosing acute exacerbation period of chronic obstructive pulmonary disease
InactiveCN102253220BEasy to measure and reliableBiological testingInterleukin-18 binding proteinAbnormal macrophage
The invention relates to a kit used for diagnosing the acute exacerbation period of chronic obstructive pulmonary disease. The kit comprises a cell factor and an enzyme labeled antibody thereof and is characterized in that the cell factor is interleukin 9, interleukin 18 binding protein A, C-C sequence ligand 28, a human skin T cell capture chemokine, a Beta cytokine, a monocyte chemoattractant protein-3, a monocyte chemoattractant protein-4, a lymphotoxin induced protein entering the T cell, a macrophage-derived chemokine, a human bone marrow suppression factor 1, a human macrophage stimulating protein, an osteopontin and a human thymus expressed chemokine. The invention firstly proposes that the cell factor can be used as a leading indicator for diagnosis of the acute exacerbation period of the chronic obstructive pulmonary disease.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com