Method for recombining, expressing and producing human thymosin in yeast
A technology of thymosin, a mature person, applied in the field of genetic engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
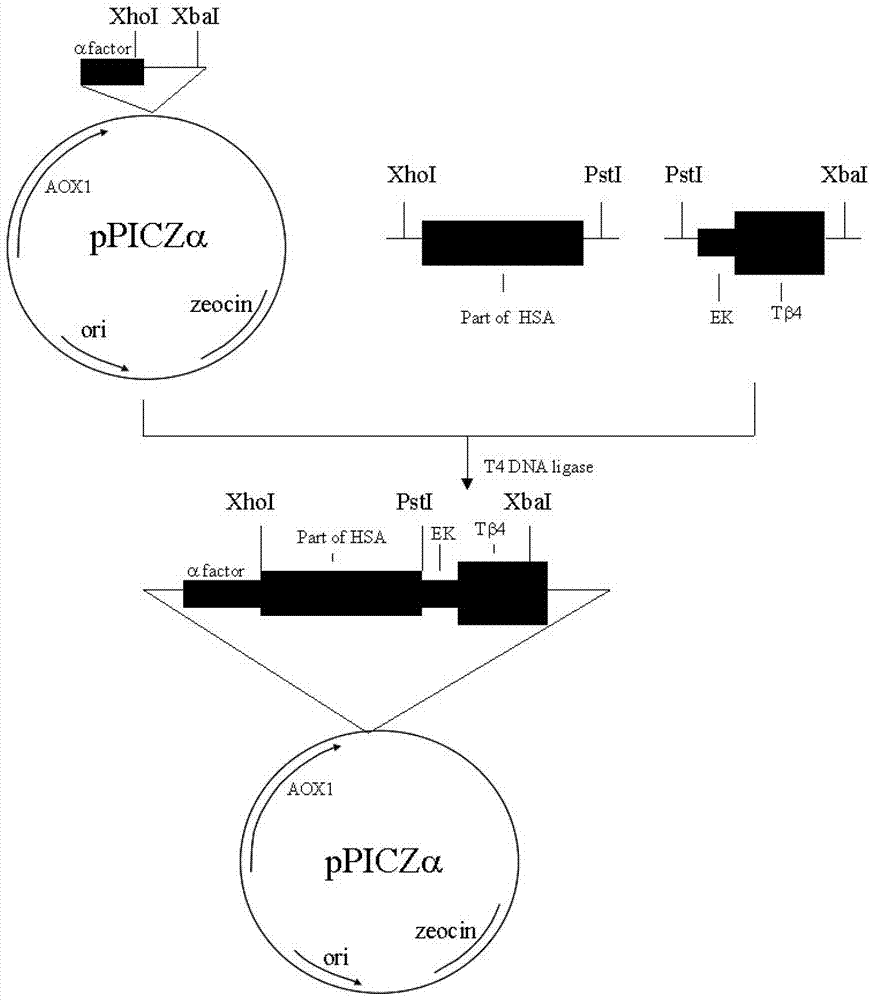
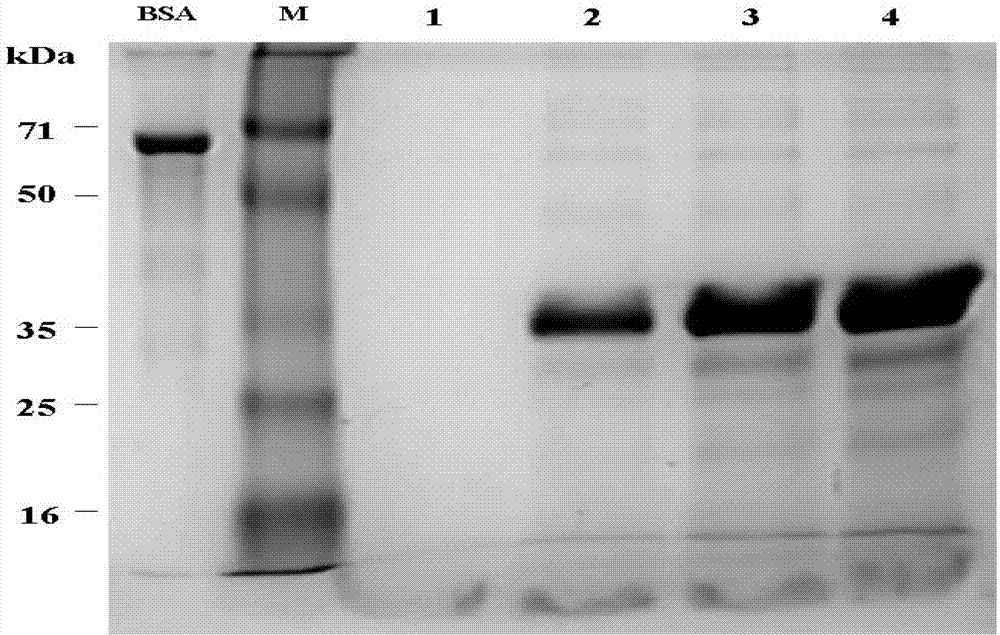
[0041] Embodiment 1: the production of recombinant human thymosin β4
[0042] Step 1. Design and biosynthesis of the N-terminal partial human serum albumin (N-HSA) gene
[0043] According to the human serum albumin gene whose sequence number is recorded as NM_000477 in GenBank or P02768 (SEQ ID No.1) in Uniprot, 2 primers were designed, and the restriction sites and protective bases of XhoI and PstI were introduced respectively. This DNA fragment contains XhoI and PstI restriction sites and encodes 186 amino acid residues of amino-terminal human serum albumin.
[0044] Upstream primer sequence: 5'-GATCAGTCTCGAGAAAAGAGATGCACACAAGAGTGAGGTTGC-3';
[0045] Downstream primer sequence: 5'-GCAGTCACTGCAGCCGAAGTTCATCGAGCTTTGGC-3';
[0046] The primers were synthesized by Shanghai Sangon Biotechnology Co., Ltd. The PCR method is: add 5 μl 10×Pfu buffer (containing MgSO4), 40 μl water, 1 μl (100ng / μl) to 50 μl system
[0047] For cDNA library, 1 μl of upstream and downstream primers ...
Embodiment 2
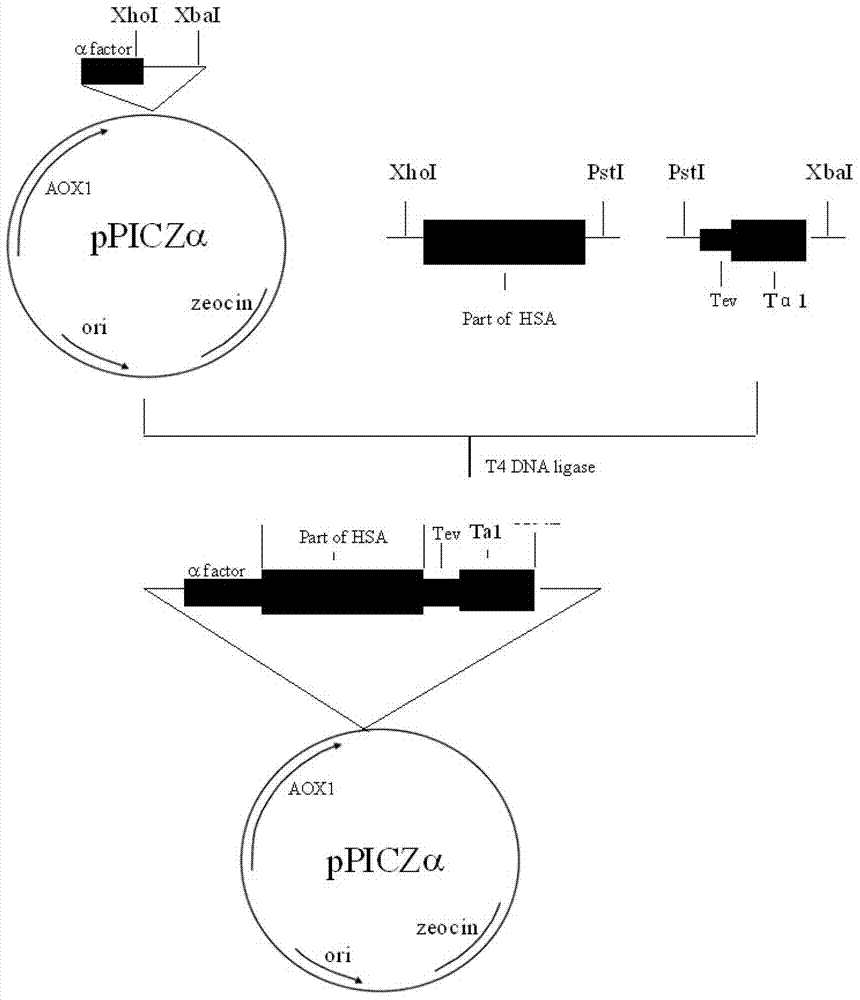
[0061] Embodiment 2: the production of recombinant human thymosin α1
[0062] Step 1. Design and biosynthesis of the N-terminal partial human serum albumin (N-HSA) gene
[0063] According to the human serum albumin gene whose sequence number is recorded as NM_000477 in GenBank or P02768 (SEQ ID No.1) in Uniprot, 2 primers were designed, and the restriction sites and protective bases of XhoI and PstI were introduced respectively. This DNA fragment contains XhoI and PstI restriction sites and encodes 186 amino acid residues of amino-terminal human serum albumin.
[0064] Upstream primer sequence: 5'-GATCAGTCTCGAGAAAAGAGATGCACACAAGAGTGAGGTTGC-3';
[0065] Downstream primer sequence: 5'-GCAGTCACTGCAGCCGAAGTTCATCGAGCTTTGGC-3';
[0066] The primers were synthesized by Shanghai Sangon Biotechnology Co., Ltd. The PCR method is: add 5 μl 10×Pfu buffer (containing MgSO4) to 50 μl system, 40 μl water, 1 μl (100 ng / μl) cDNA library, 1 μl (5 μmol / L) of upstream and downstream primers, 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com