Liquid formulation comprising anti-human thymic stromal lymphopoietin (tslp) monoclonal antibody
A monoclonal antibody, liquid preparation technology, applied in non-active ingredients medical preparations, anti-animal/human immunoglobulins, antibodies, etc., can solve the process failure, concentration polarization out of control, the sliding performance of the charging syringe Reduce and other problems to achieve good clinical results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
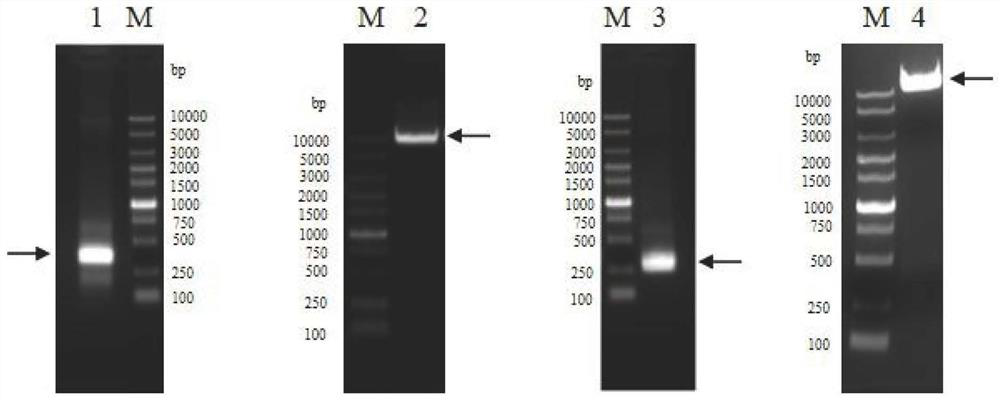
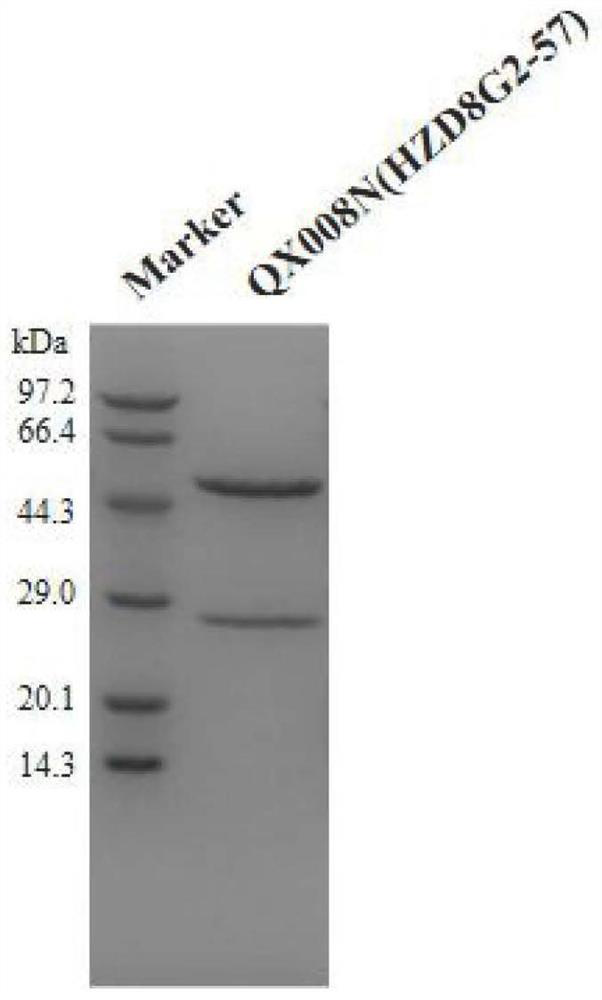
[0127] Example 1 Preparation of anti-human TSLP monoclonal antibody QX008N
[0128] Procured human thymic stromal lymphopoietin (hTSLP) from Shanghai Nearshore Technology Co., Ltd., used to immunize New Zealand rabbits, used B cell cloning technology to obtain antigen-binding specific antibody clones, and then screened out binding to human TSLP and having human TSLP inhibitory activity of monoclonal antibodies. Cell supernatants were detected by Binding ELISA and Blocking ELISA to select target clones. The above immunization and screening processes are entrusted to commercial companies.
[0129] Seven clones were selected for recombinant expression and sequenced. It was determined that 8G2 had the best cell neutralization activity. Therefore, the 8G2 clone was humanized. Human IgG germline sequence (Germline) homology alignment was performed using NCBI IgBlast, IGHV3-66*01 was selected as the heavy chain CDR transplantation template, and the CDR region of the heavy chain (...
Embodiment 2
[0134] Example 2 Determination of Equilibrium Dissociation Constant (KD)
[0135] The affinity of QX008N (HZD8G2-57) to human TSLP was detected by Biacore T200, all at 25°C. A commercial Protein A chip was used, and an appropriate amount of antibody was immobilized by the capture method, so that the Rmax was around 50RU, and the capture flow rate was 10 μl / min. The antigen was serially diluted, the flow rate of the instrument was switched to 30 μl / min, and the concentration flowed through the reference channel and the immobilized antibody channel in order of concentration from low to high, and the buffer was used as a negative control. The chip was regenerated with pH 1.5 glycine after each binding and dissociation was completed. Use the built-in analysis software of the instrument to select the 1:1 binding model in the Kinetics option for fitting, and calculate the association rate constant ka, dissociation rate constant kd and dissociation equilibrium constant KD value of t...
Embodiment 3
[0140] Example 3 QX008N and Tezepelumab neutralize the STAT5 phosphorylation activity of human TSLP-induced SW756-STAT5-Luciferase reporter gene cells. The SW756-STAT5-Luciferase reporter gene cell line was used to determine that QX008N antagonized human TSLP through TSLPR-IL-7R-mediated intracellular Phosphorylation activity of the signaling molecule STAT5: cells in the culture medium were plated at 4 × 10 per well 4 Cells were added to 96 wells and then incubated at 37°C and 5% CO 2 Incubate overnight under conditions. A pre-incubated mixture of antibody and human TSLP was added to the cells in a final concentration range of 0 to 50 ng / ml for QX008N, 0 to 400 ng / ml for Tezepelumab, and 0.5 ng / ml for TSLP. Then at 37°C and 5% CO 2 Incubate for 24 hours under conditions, discard the cell culture supernatant, add 120 μl ONE-Glo-Luciferase Reagent to each well for 30 min, take 100 μl from each well to a white 96-well plate, detect the Luminescence fluorescence signal value and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com