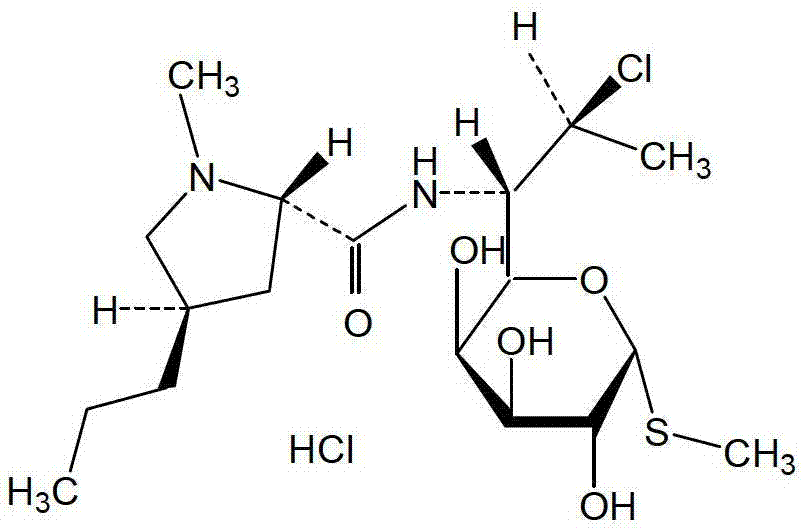
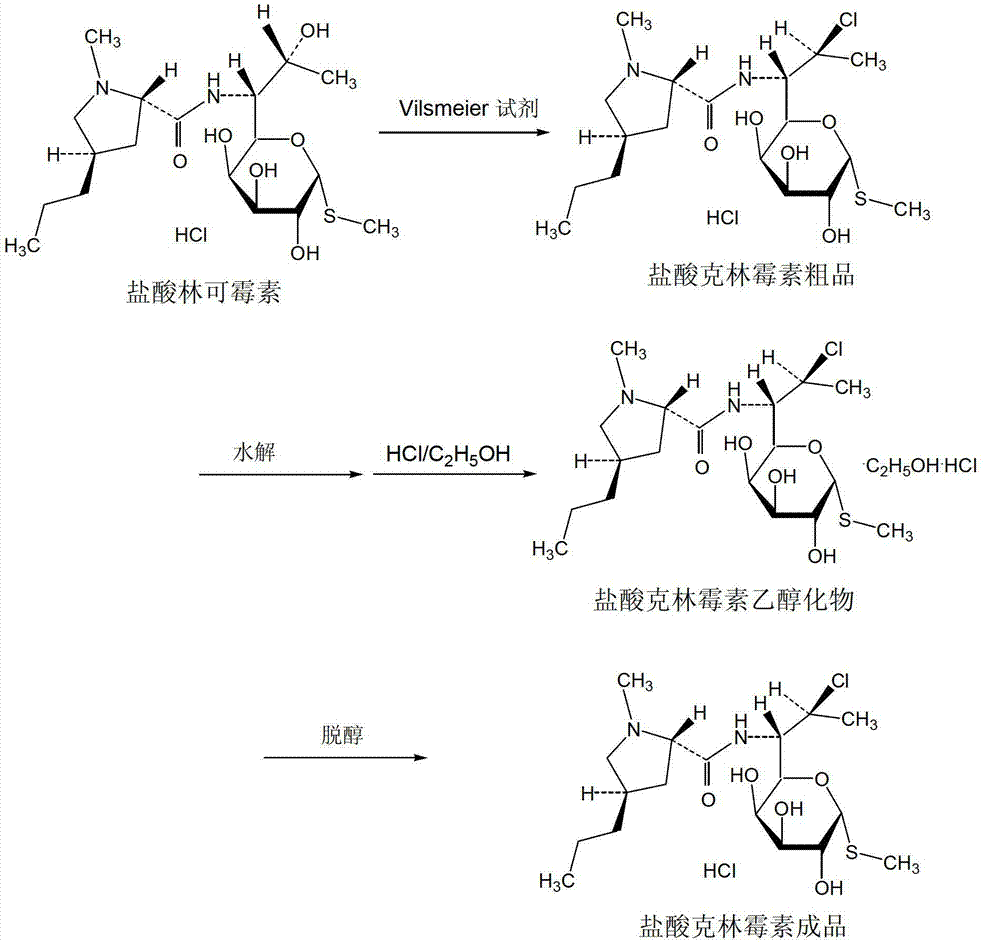
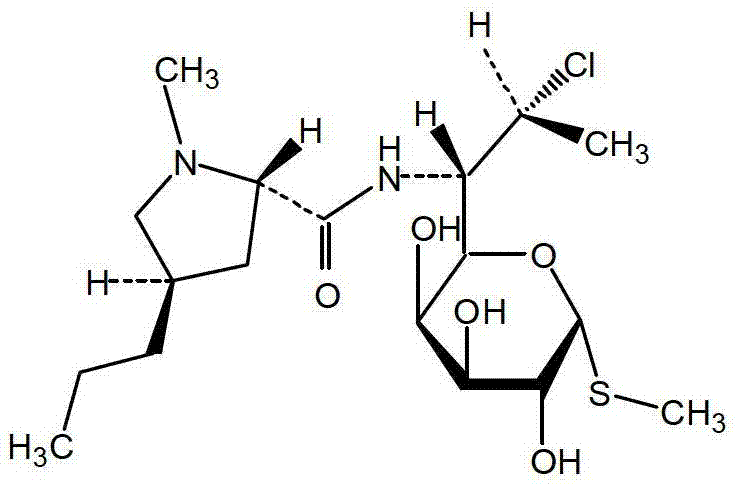
Method for preparing clindamycin hydrochloride
A technology of clindamycin hydrochloride and lincomycin hydrochloride, applied in the field of medicinal chemistry, can solve the problems of high content of related substances, long reaction time, inability to meet the requirements of high-end customers, etc., to reduce the content of impurities and avoid easy absorption The effect of tide and process optimization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1 Preparation of clindamycin hydrochloride
[0071] Add 90kg of chloroform, 16kg of DMF and 10kg of lincomycin hydrochloride (HPLC purity 99.6%) into the reaction kettle in turn, cool to 0°C, add 10kg of solid phosgene in batches, control the temperature in the kettle not to exceed 15°C when adding, and then Raise the temperature to 40°C for 15 hours, and then react at 65°C for 3 hours. HPLC detects that the content of lincomycin hydrochloride in the reaction system is less than 1.0%, and the reaction is complete.
[0072] Cool the above reaction solution to 20°C, add 10% sodium hydroxide aqueous solution dropwise, adjust the pH to 9, control the temperature of the system not to exceed 30°C, keep it warm for 2 hours, let it stand for layers, extract the water phase once with 70kg of chloroform, and combine The organic phase was washed once with 20 L of water, and the organic phase was concentrated in vacuum below 80° C. until no liquid droplets appeared to ob...
Embodiment 2
[0076] Example 2 Preparation of clindamycin hydrochloride
[0077] Add 120kg of chloroform, 20kg of DMF and 10kg of lincomycin hydrochloride (HPLC purity 99.6%) into the reaction kettle in turn, cool to 0°C, add 13kg of solid phosgene in batches, control the temperature in the kettle not to exceed 15°C when adding, and then The temperature was raised to 50°C for 15 hours, and then at 55°C for 5 hours. HPLC detects that the content of lincomycin hydrochloride in the reaction system is less than 1.0%, and the reaction is complete.
[0078] Cool the above reaction solution to 20°C, add 10% sodium hydroxide aqueous solution dropwise, adjust the pH to 12, control the temperature of the system not to exceed 30°C, keep it warm for 2 hours, let it stand for stratification, extract the water phase once with 70kg of chloroform, and combine The organic phase was washed once with 20 L of water, and the organic phase was concentrated in vacuum below 80° C. until no liquid droplets appe...
Embodiment 3
[0082] Example 3 Preparation of clindamycin hydrochloride
[0083] Add 55kg of chloroform, 16kg of DMF and 10kg of lincomycin hydrochloride (HPLC purity 99.5%) into the reaction kettle in sequence, cool to 0°C, add dropwise a solution of 11kg of solid phosgene dissolved in 25kg of chloroform, and finish adding in 3 hours. The temperature in the kettle does not exceed 10°C, and then the temperature is raised to 45°C for 10 hours, and then reacted at 60°C for 4 hours. When the content of lincomycin hydrochloride in the reaction system detected by HPLC is less than 1.0%, the reaction is complete.
[0084] Cool the above reaction solution to 20°C, add 10% sodium hydroxide aqueous solution dropwise, adjust the pH to 10, control the temperature of the system not to exceed 30°C, keep it warm for 2 hours, let it stand for stratification, extract the water phase once with 70kg of chloroform, and combine The organic phase was washed once with 20 L of water, and the organic phase was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com