Long-acting human endothelium chalone containing immune globulin Fc segment
A technology of immunoglobulin and endostatin, applied in the direction of peptide/protein components, medical preparations containing active ingredients, medical preparations with non-active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The preparation of embodiment 1.rhES-PEG
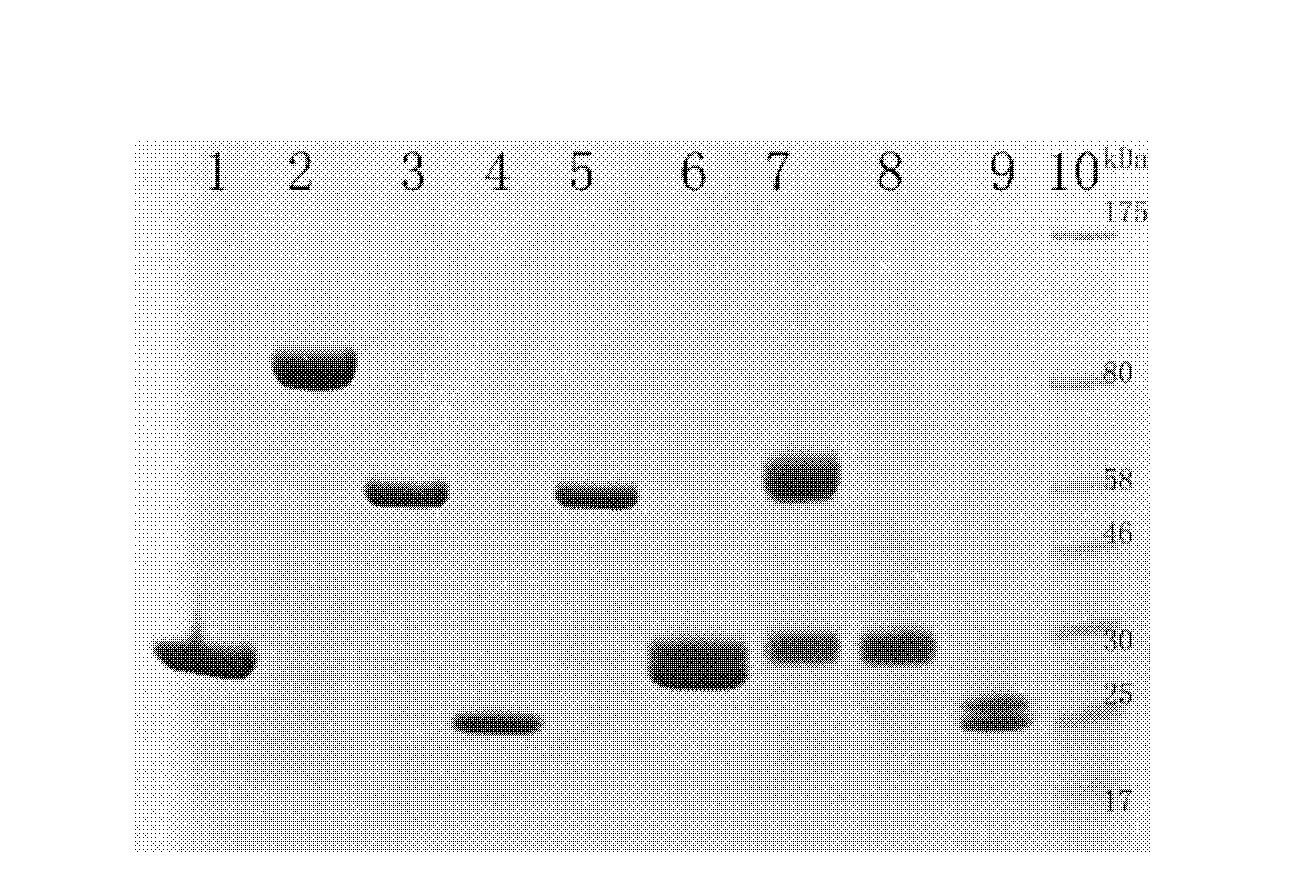
[0055] 3.4kDa polyethylene glycol (ALD-PEG-ALD) with aldehyde groups at both ends and recombinant human endostatin rhES (from Jiangsu Xiansheng Mai De Jin Biopharmaceutical Co., Ltd., product batch number 20100305, the sequence is as follows figure 2 shown) was dissolved in 2M sodium acetate buffer at a molar ratio of 15:1, and the reducing agent sodium cyanoborohydride with a final concentration of 20 mM was added to the mixture, and reacted at 4 ° C for 1 h under gentle stirring to make PEG and The N-terminal amino group of rhES is linked.
[0056] The final product of the reaction was purified using Source S (purchased from GE Healthcare, Source 15S, Cat. No. 17-0944-03). The above reaction mixture was diluted 20-fold with 50 mM, pH 6.4 4-morpholineethanesulfonic acid (MES) buffer and loaded. The elution buffer is 50mM MES buffer containing 1M NaCl, and the gradient concentration is used for elution. The gradient is that...
Embodiment 2
[0057] Example 2. Preparation of rhES-PEG-Fc
[0058] The mono-PEGylated rhES purified in Example 1 above was further coupled to the N-terminus of the Fc fragment of the immunoglobulin.
[0059] The Fc fragment (non-glycosylated human IgG4 Fc fragment expressed by Escherichia coli BL21 (DE3), NCBI Gene ID: 3503. Obtained from Beijing Hanmei Pharmaceutical Co., Ltd.) and the rhES-PEG at a molar ratio of 15:1 Dissolve in 50mM, pH5.5 sodium acetate buffer, add the reducing agent sodium cyanoborohydride at a final concentration of 20mM to this mixture, and react at 4°C for 16h under gentle stirring to make the rhES-PEG and Fc fragment The N-terminal coupling.
[0060]The final product of the reaction was purified using Source S (purchased from GE Healthcare, Source 15S, Cat. No. 17-0944-03). The above reaction mixture was diluted 20-fold with 50 mM sodium acetate buffer, pH 5.5, and then loaded. The elution buffer was 50 mM sodium acetate buffer containing 1 M NaCl. Elute with...
Embodiment 3
[0064] Example 3. Determination of anti-vascular endothelial cell migration activity of rhES-PEG-Fc in vitro
[0065] In this example, the anti-migration activity of rhES-PEG-Fc of the present invention on human microvascular endothelial cells (HMVEC) was tested.
[0066] HMVEC cells in the logarithmic growth phase (purchased from ATCC, product number CRL-4025) were starved overnight, and inoculated with 8×10 4 The cells were placed in a culture dish Transwell (purchased from Corning, Cat. No. 3422) for detecting cell migration. The Transwell culture dish is a culture chamber with a permeable support, and the bottom of the chamber is a permeable membrane, that is, the permeable support. In this embodiment, a Transwell culture dish with a PC membrane with a pore size of 8.0 μm is used.
[0067] Establish negative control group (N.C. group, serum-free medium DMEM), positive control group (P.C. group, add 2% fetal bovine serum FBS and 10ng / ml vascular endothelial growth factor ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com