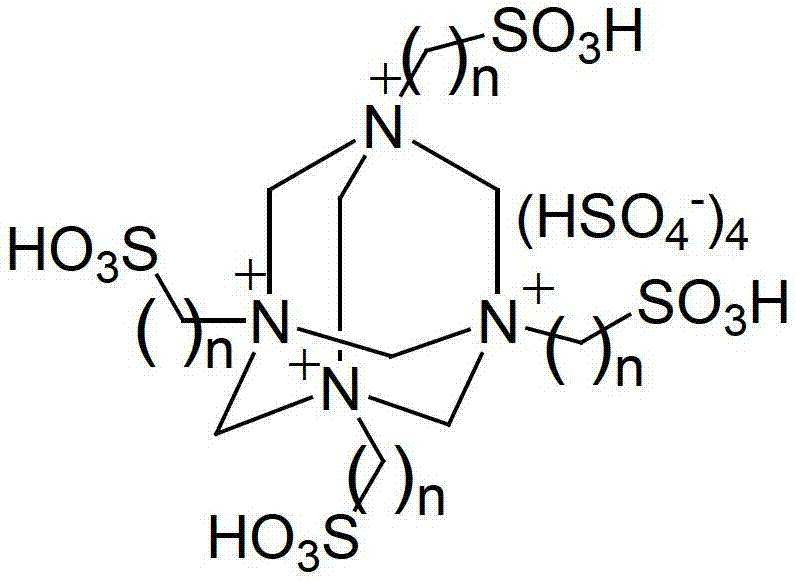
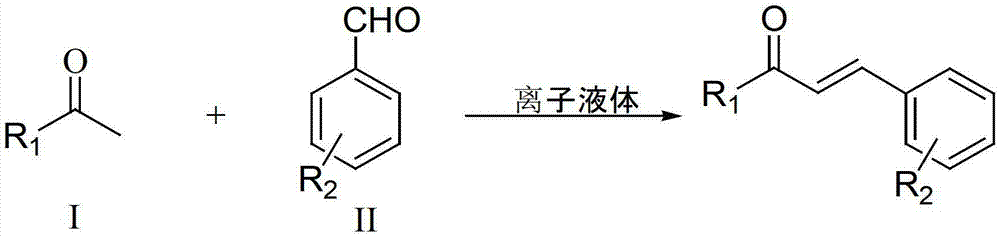
Method for preparing chalcone and directive through multi-sulfonate ion liquid catalysis
A technology of ionic liquid and polysulfonate, which is applied in the preparation of carbon-based compounds, chemical instruments and methods, and the preparation of organic compounds, etc., can solve the difficulties of product separation, environmental pollution, catalyst usage, and large loss in recycling, etc. problem, to achieve the effect of high catalytic activity, convenient separation and high acid density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: under nitrogen protection, 10mmol benzaldehyde, 10mmol acetophenone and 0.8mmol IL-SO 3 H-a was added to a 50mL three-necked flask with a stirring bar and a reflux condenser. Vigorously stir and heat up to 120°C, react for 8 hours, cool to room temperature, the reaction solution is divided into two layers after standing, the upper layer solution is separated by decantation, recrystallization and purification can obtain 1,3-diphenyl-2-propene-1 - Ketone (chalcone) yield was 94%. Contains IL-SO 3 The lower layer solution of H-a can be recycled after being vacuum-dried at 110°C for 2 hours.
Embodiment 2
[0020] Embodiment 2: under nitrogen protection, 10mmol4-methoxybenzaldehyde, 10mmol acetophenone and 1mmol IL-SO 3 H-a was added to a 50mL three-necked flask with a stirring bar and a reflux condenser. Vigorously stir and heat up to 140°C, react for 9 hours, cool to room temperature, the reaction solution is divided into two layers after standing, and the upper layer solution is separated by decantation, recrystallized and purified to obtain 4-methoxychalcone with a yield of 90% . Contains IL-SO 3 The lower layer solution of H-a can be recycled after being vacuum-dried at 110°C for 2 hours.
Embodiment 3
[0021] Embodiment 3: under argon protection, 10mmol3-chlorobenzaldehyde, 10mmol acetophenone and 1mmol IL-SO 3 H-b was added to a 50mL three-neck flask with a stirring bar and a reflux condenser. Stir vigorously and raise the temperature to 140°C, react for 8 hours, and cool to room temperature. The reaction solution is separated into two layers after standing, and the upper layer solution is separated by decantation, and the yield of 3-chlorochalcone can be obtained by recrystallization and purification. The yield is 87%. Contains IL-SO 3 The lower layer solution of H-b can be recycled after being vacuum-dried at 110°C for 2 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com