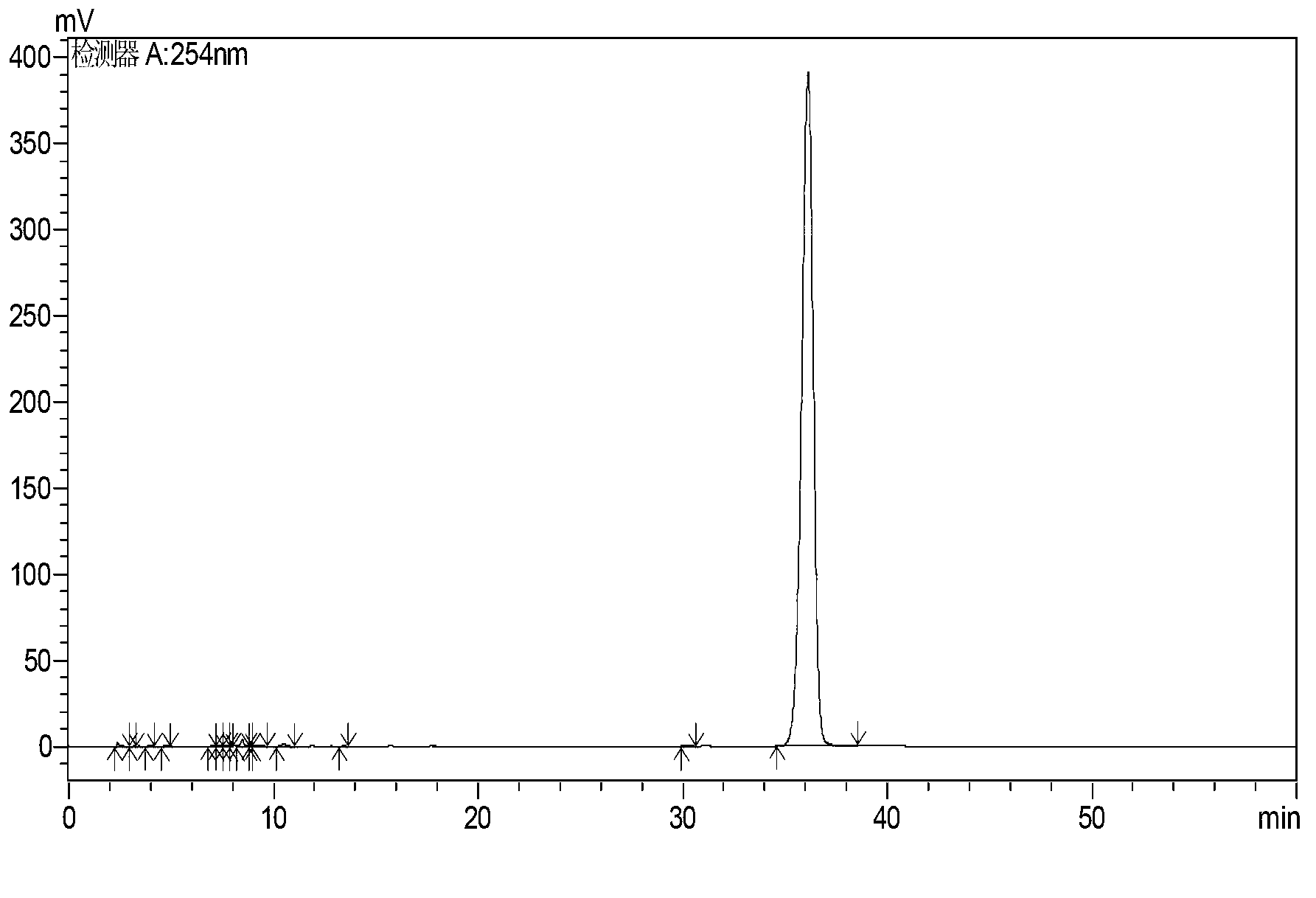
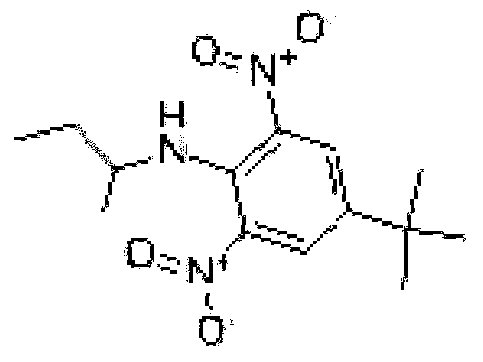
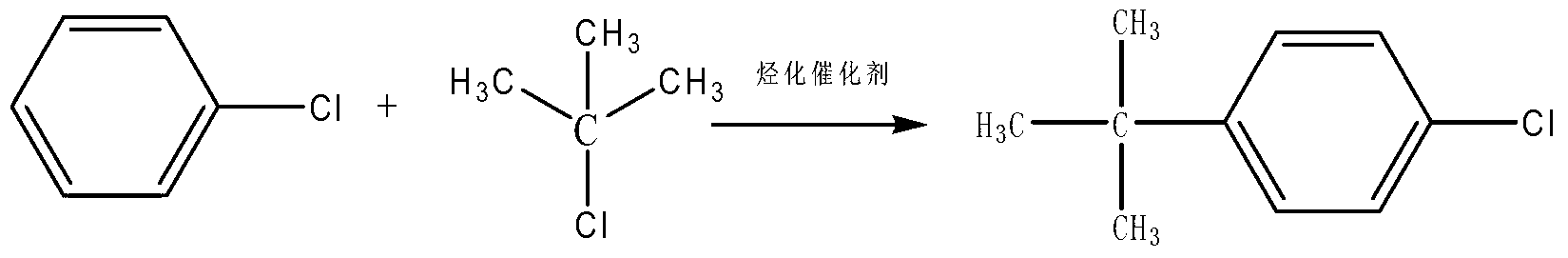
Synthesis method of high-purity butralin
A synthesis method and high-purity technology, applied in chemical instruments and methods, preparation of amino compounds, preparation of organic compounds, etc., can solve the problems of low purity of butadiene, and achieve the effect of simple synthesis method and improved purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0021] Specific embodiment one: the synthetic method of the high-purity Zhongbutaline of the present embodiment realizes according to the following steps:
[0022] 1. Add 295-305g of dichloroethane and 35-40g of p-tert-butylphenol into a four-necked flask equipped with a condenser, heat to 45-55°C, then add 45-50g of nitric acid solution dropwise, and react for 12- For 18 minutes, continue to heat up to 68-72°C, then transfer to a separatory funnel, let stand to separate the upper layer of acid water to obtain the mononitrated organic phase, and then transfer the mononitrated organic phase to a four-necked flask equipped with a condenser. Heat to 45-55°C, add 22-26g of concentrated nitric acid dropwise, react for 12-18 minutes, continue to heat up to 68-72°C, then transfer to a separatory funnel, let stand to separate the upper layer of acid water, and obtain the dinitrated organic phase , transfer the dinitrated organic phase into a four-necked bottle with a condensation wate...
specific Embodiment approach 2
[0033] Specific embodiment 2: The difference between this embodiment and specific embodiment 1 is that the time used for adding 48.6 g of nitric acid solution dropwise in step 1 is 45 minutes. Other steps and parameters are the same as those in Embodiment 1.
specific Embodiment approach 3
[0034] Specific embodiment three: the difference between this embodiment and specific embodiment one or two is that step one takes 30 minutes to add 24.3 g of concentrated nitric acid dropwise. Other steps and parameters are the same as those in Embodiment 1 or Embodiment 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com