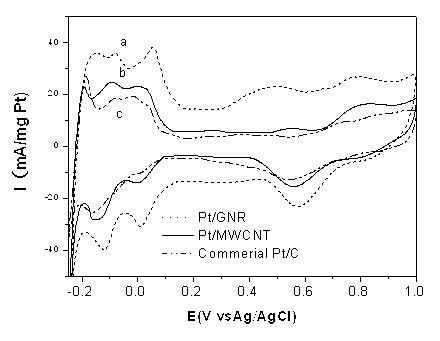
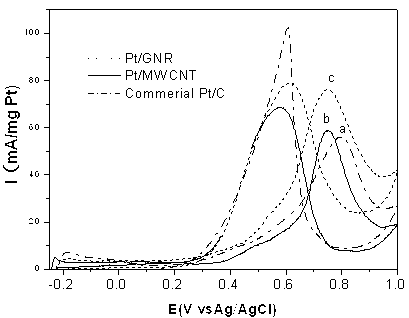
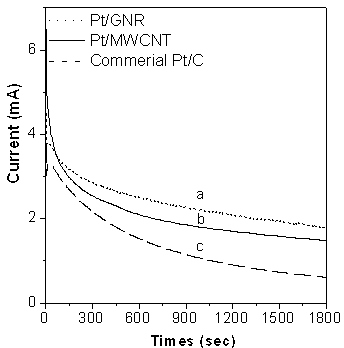
Band-shaped carbon-carrier metal catalyst, preparation method and application thereof
A metal catalyst, catalyst technology, applied in metal/metal oxide/metal hydroxide catalysts, catalyst supports, chemical instruments and methods, etc., can solve chemical corrosion, catalyst durability reduction, nano-carbon aerogel preparation Difficulties and other problems to achieve the effect of prolonging life, preventing agglomeration, and improving the ability to resist carbon monoxide poisoning
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Put 1 gram of carbon nanotubes in 100ml of 60% H 2 SO 4 , stirred at 70°C for 1 h to prepare a sulfuric acid solution containing carbon nanotubes;
[0032] (2) 5 g of KMnO 4 Add it into the sulfuric acid solution containing carbon nanotubes, and continue to stir for 1h to obtain carbon nanoribbons containing oxidized cuts, KMnO 4 and a mixture of sulfuric acid;
[0033] (3) Add H to the mixture in step (2) in sequence 2 o 2 Centrifuge with hydrochloric acid and deionized water, wash the precipitate several times, and dry it in a vacuum oven at 50°C for 12 hours to obtain graphene oxide nanobelts;
[0034] (4) Put the graphene oxide nanoribbon into deionized water, ultrasonic for 30 minutes, then add chloroplatinic acid, palladium acetate, nickel acetate, the content of platinum in the catalyst metal is 10%, and the content of palladium and nickel is respectively 5%, After stirring for 20 minutes, add 0.1 g of sodium borohydride, perform a reduction reaction at...
Embodiment 2
[0036] (1) Put 1 gram of carbon nanotubes in 100ml 98%H 2 SO 4 , stirred at 80°C for 2 hours to prepare a sulfuric acid solution containing carbon nanotubes;
[0037] (2) 5 g of KMnO 4 Added to the sulfuric acid solution containing carbon nanotubes, and continued to stir for 0.5 h, to obtain 4 and a mixture of sulfuric acid;
[0038] (3) Add H to the mixture in step (2) in sequence 2 o 2 Centrifuge with hydrochloric acid and deionized water, wash the precipitate several times, and dry it in a vacuum oven at 50°C for 12 hours to obtain impurity-free carbon nanoribbons;
[0039] (4) Put the impurity-free nano-carbon ribbon into methanol, ultrasonicate for 20 minutes, then add chloroplatinic acid and manganese acetate solution, the platinum content in the catalyst is 20%, the manganese content is 4%, after stirring for 20 minutes, 120 Reduction reaction at ℃ for 10 h, filtered, vacuum-dried, and cooled at room temperature to obtain the strip-shaped carbon-supported metal ca...
Embodiment 3
[0041] (1) Put 1 gram of carbon nanotubes in 100ml H 3 PO 4 , 40%H 2 SO 4 , stirred at 90°C for 1 h to prepare a sulfuric acid solution containing carbon nanotubes;
[0042] (2) 5 g of KMnO 4 Add to the sulfuric acid solution containing carbon nanotubes, continue to stir for 3h, and obtain carbon nanoribbons containing oxidized cuts, KMnO 4 , a mixture of sulfuric acid;
[0043] (3) Add H to the mixture in step (2) in sequence 2 o 2 Centrifuge with hydrochloric acid and deionized water, wash the precipitate several times, and dry it in a vacuum oven at 50°C for 12 hours to obtain impurity-free carbon nanoribbons;
[0044] (4) Put the impurity-free nanocarbon ribbon into ethylene glycol, ultrasonicate for 20 minutes, then add platinum platinum acid and ferric chloride solution, the platinum content in the catalyst is 30%, and the iron content After stirring at 10% for 20 minutes, reduction reaction at 120°C for 24 hours, filtration, vacuum drying, and cooling at room te...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com