Polygeline dextran composition
A technology of polygelatin peptide and plasma replacement, which is applied in the field of medicine, can solve problems such as potential safety hazards, and achieve the effects of high safety, accurate electrolyte control, and reliable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
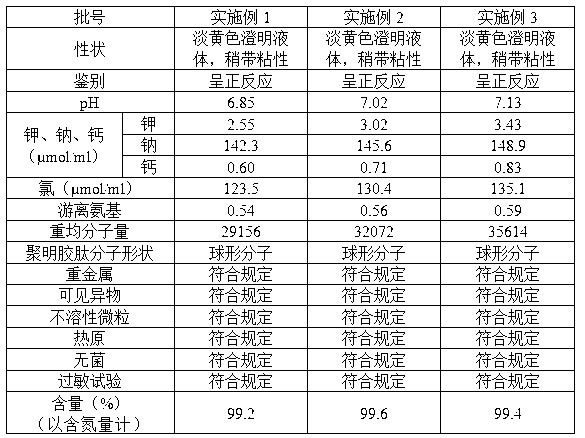
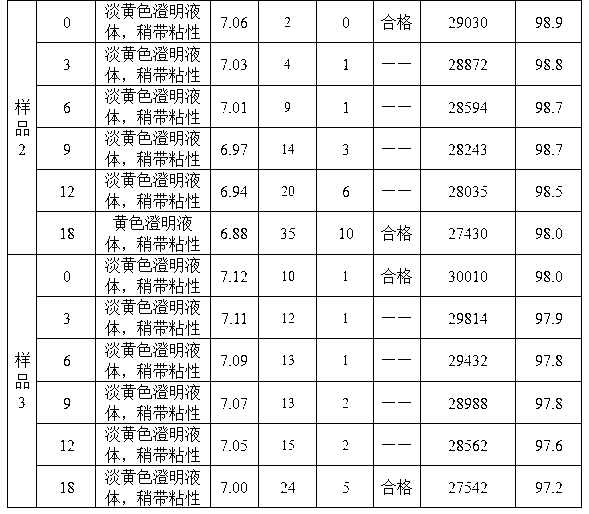
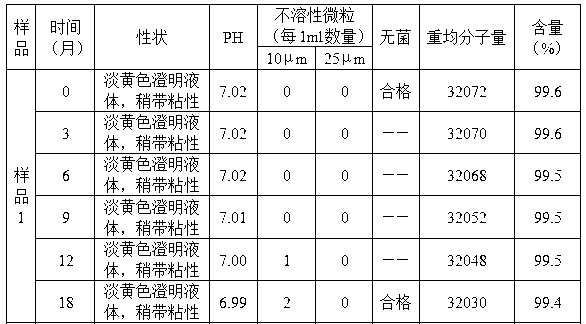
Embodiment 1
[0029] 1. Dissolve 190g of commercially available bovine bone gelatin (32g in terms of nitrogen content) with 2250ml of water for injection at 60°C, adjust the pH value to 5.0 with hydrochloric acid solution or sodium hydroxide, place the gelatin solution at a power of 0.8KW, and a frequency of Under the ultrasonic field of 40KHz, the temperature was raised to 78°C, and the temperature was maintained. When the relative viscosity reached 1.65, the ultrasonic was stopped, cooled to 25°C, and the gelatin degradation solution was obtained;
[0030] 2. Use sodium hydroxide to adjust the pH of the degradation solution to 7.6, add 2g of glutaraldehyde at 25°C, stir continuously, crosslink for 1 hour, adjust the pH to 9.5 with sodium hydroxide, and add 2g of glutaraldehyde at 32°C Add 1.8g of trimethylolpropane, stir continuously, and cross-link for 1.5 hours; adjust the pH value to 11.0 with sodium hydroxide, and stir continuously at 29°C, and cross-link for 0.8 hours;
[0031] 3. Ad...
Embodiment 2
[0034] 1. Dissolve 205g of commercially available bovine bone gelatin (32g in terms of nitrogen content) in 2250ml of water for injection at 70°C, adjust the pH value to 6.0 with hydrochloric acid solution or sodium hydroxide, place the gelatin solution at a power of 0.8KW, and a frequency of Under the ultrasonic field of 40KHz, the temperature was raised to 75°C, and the temperature was maintained until the relative viscosity reached 1.80, the ultrasonic was stopped, cooled to 25°C, and the gelatin degradation solution was obtained;
[0035] 2. Use sodium hydroxide to adjust the pH of the degradation solution to 8.6, add 2.2g of glutaraldehyde at 30°C, stir continuously, crosslink for 1 hour, adjust the pH to 10.5 with sodium hydroxide, and add 2.2g of glutaraldehyde at 37°C Add 2.1 g of trimethylolpropane, stir continuously, and cross-link for 1 hour; adjust the pH value to 11.5 with sodium hydroxide, and stir continuously at 34°C, and cross-link for 1 hour;
[0036] 3. Add ...
Embodiment 3
[0039] 1. Dissolve 210g of commercially available pork bone gelatin (32g in terms of nitrogen content) in 2250ml of water for injection at 62°C, adjust the pH value to 5.8 with hydrochloric acid solution or sodium hydroxide, place the gelatin solution at a power of 0.8KW, and a frequency of Under the ultrasonic field of 40KHz, the temperature was raised to 80°C, and the temperature was maintained, and when the relative viscosity reached 1.75, the ultrasonic was stopped, cooled to 26°C, and the gelatin degradation solution was obtained;
[0040] 2. Use sodium hydroxide to adjust the pH of the degradation solution to 8.4, add 2.3g of glutaraldehyde at 28°C, stir continuously, crosslink for 1 hour, adjust the pH to 10.0 with sodium hydroxide, and add 2.3g of glutaraldehyde at 37°C 2.2 g of trimethylolpropane was added under constant stirring, and cross-linked for 1 hour; the pH value was adjusted to 11.2 with sodium hydroxide, and at 32° C., stirred continuously, and cross-linked ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com