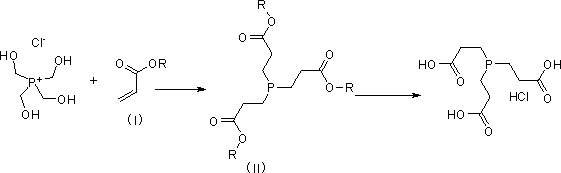
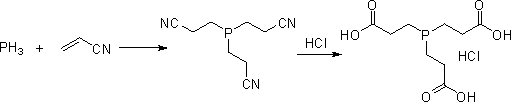
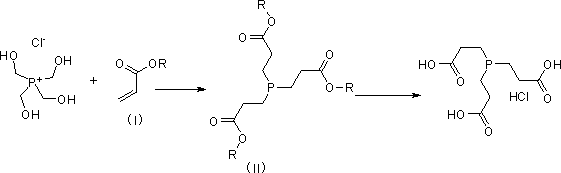
Preparation method of tris (2-carboxyethyl) phosphine hydrochloride
A technology of phosphorus hydrochloride and carbonyl ethyl, which is applied in the field of preparation of triphosphorus hydrochloride, can solve the problems of low product purity and inability to achieve effective separation, and achieve high product purity, strong route feasibility, and low toxicity of raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1.1 Preparation of compound trimethyl 3, 3', 3''-phosphinetriyltripropionate
[0031] Under the condition of mechanical stirring, add tetrakis hydroxymethyl phosphorus chloride aqueous solution (71.2g, 0.30mol) and ethanol (75ml) with a mass fraction of 80% to a 250ml reaction flask, cool with ice water and protect with nitrogen, add hydroxide Potassium (16.8g, 0.30mol) was dissolved in 23ml of ice water, and the temperature rose slightly; then ethyl acrylate (90g, 0.9mol) was added dropwise, and the temperature was controlled at 35-40°C, a large amount of solids were formed, keep stirring after dropping 4 hours. Pour 300ml of ice water into the reaction solution, stir and filter with suction, wash the filter cake with ice water, and dry it with suction to obtain 88g of white solid (that is, the compound trimethyl 3, 3', 3''-phosphinetriyltri propionate), yield 86.6%, for the next step reaction. The NMR data of the resulting product are as follows:
[0032] 1 ...
Embodiment 2
[0037] 2.1 Preparation of compound trimethyl 3, 3', 3''-phosphinetriyltripropionate
[0038] Under the condition of mechanical stirring, add tetrakis hydroxymethyl phosphorus chloride aqueous solution (71.2g, 0.30mol) and tetrahydrofuran (75ml) with a mass fraction of 80% into a 250ml reaction flask, cool with ice water and protect with nitrogen, add sodium hydroxide (12g, 0.30mol) dissolved in 23ml of ice water solution, the temperature rose slightly. Then ethyl acrylate (126g, 1.26mol) was added dropwise, and the temperature was controlled at 55-60°C. A large amount of solids were formed, and the mixture was kept stirring for 6 hours after dropping. Pour 300ml of ice water into the reaction solution, stir and filter with suction, wash the filter cake with ice water, and dry it with suction to obtain 94g of white solid, (that is, the compound trimethyl 3, 3', 3''-phosphine triyl Tripropionate), yield 92.5%, is used for next step reaction. The NMR data of the resulting p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com