Gametophyte antigen gam22 gene of Eimeria necatrix and application of gene
A technology of Eimeria and antigen genes, applied in application, gene therapy, genetic engineering, etc., can solve the problem of no gene sequence recombination expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
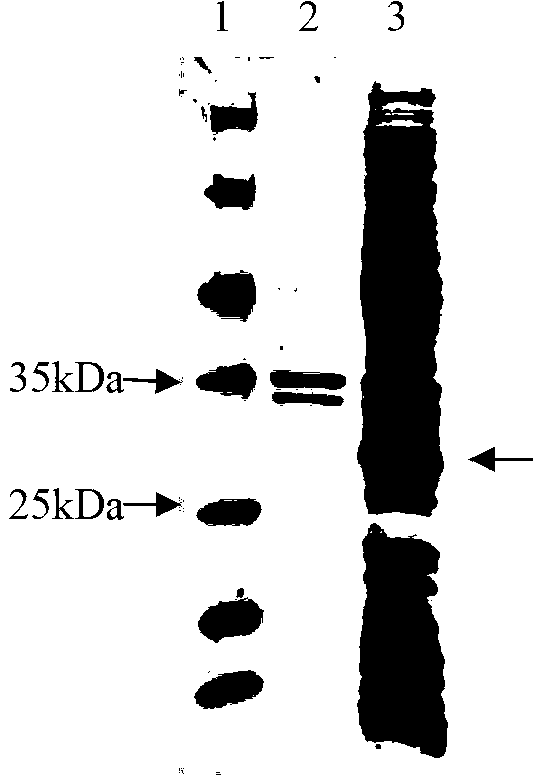
Image
Examples
Embodiment 1
[0045] Example 1: Isolation of the gam22 gene from the Eimeria gametocyte gene
[0046] Conventional methods were used to separate and purify the gametocytes of Eimeria venomosa, and the total RNA was extracted with an RNA extraction kit.
[0047] RT-PCR catch gam22 gene:
[0048] Refer to TaKaRa company (TaKaRa RNA LA PCR TM The method provided by Kit (AMV) Ver.1.1): two-step RT-PCR kit to amplify the gam22 gene.
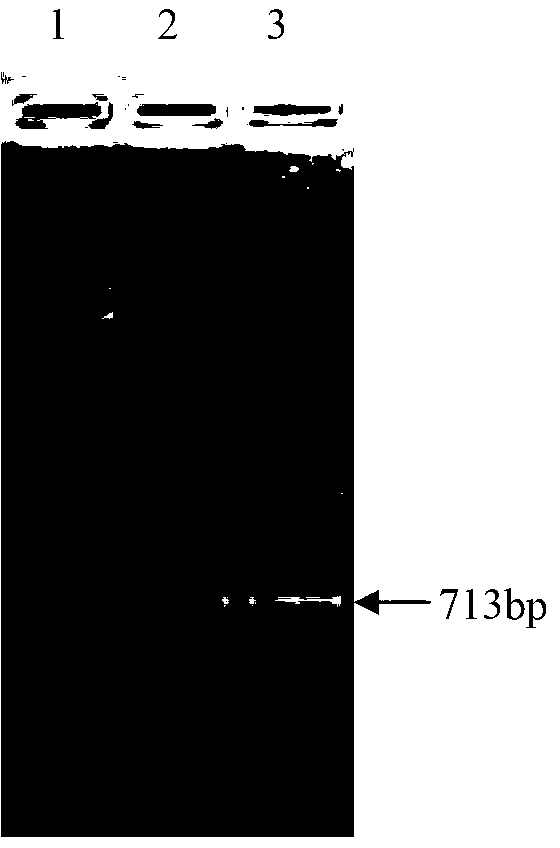
[0049] The Engam22 gene was captured using an upstream primer (5'-ACCCCAAAATAAAATCAAAGGC-3') and a downstream primer (5'-CCATGAAGATCTCAGACGTAGC-3').
[0050] The first step: reverse transcription reaction, the total system is 10μl: 0.5μl total RNA (≤500ng total RNA), 25mM MgCl 2 2μl, 10×RNA PCR Buffer1μl, RNase Free dH 2 O4.25μl, concentration of 10mM dNTP1μl, RNase Inhibitor0.25μl, 5u / μl AMV reverse transcriptase 0.5μl and Oligo dT linker primer 0.5μl. Reaction steps: reverse transcription at 42°C for 30 minutes; denaturation at 99°C for 5 minutes; cooling at...
Embodiment 2
[0052] Embodiment 2: the amplification of expression fragment Engam22
[0053] According to the ORF sequence of the Eimeria virulence gam22 gene and the restriction site of the plasmid pET28a (purchased from Invitrogen), after removing the signal peptide, specific primers were designed to amplify the gene fragment for expression.
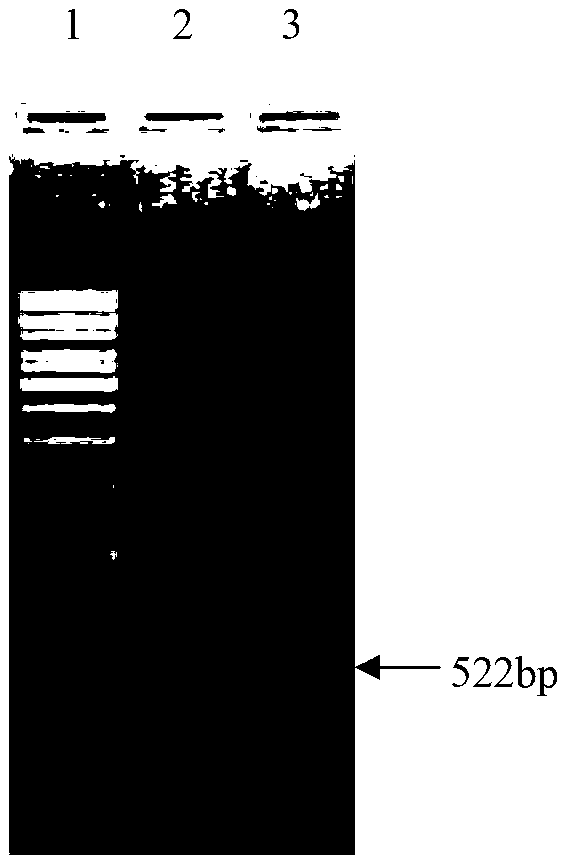
[0054] According to the enzyme cutting sites on the plasmid pET28a, after sequence analysis of the Engam22 gene, the signal peptide was eliminated, EcoR I and Hind III enzyme cutting sites were selected, and the specific primers were designed as: upstream primer (5'-TCGGAATTCGACGGAGCACCTGAG -3') contains an EcoR I restriction site and 3 protective bases, and the downstream primer (5'-GCGAAGCTTTTAGTTGATGTCGGT-3') contains a HindⅢ restriction site and 3 protective bases.
[0055] The 50 μl PCR reaction system is as follows: 5 μl of 10× reaction buffer; 1 μl of upstream and downstream primers with a concentration of 10 μM, 4 μl of dNTP (10 mM); 35 μl o...
Embodiment 3
[0056] Embodiment 3: the construction of prokaryotic expression vector
[0057] Double digestion of pET28a:
[0058] EcoRI and HindⅢ are Fermentas products. The 50 μl digestion system is: 5 μl 10× FastDigest Green Buffer, 20 μl vector pET28a, 2.5 μl each of FastDigest EcoRI and FastDigest HindⅢ, 20 μl ddH 2 O. React at 37°C for 2 hours, electrophoresis on a 1.2% agarose gel, and recover the product from the gel.
[0059] The PCR product purified in double enzyme digestion embodiment 2:
[0060] EcoRI and HindⅢ are Fermentas products. 100 μl enzyme digestion system: 10 μl 10× FastDigest Green Buffer; 20 μl purified PCR (Engam22) product; 5 μl each of FastDigest EcoRI and FastDigest HindⅢ; 60 μl ddH 2 O. React at 37°C for 2 hours, electrophoresis on a 1.2% agarose gel, and recover the digested product.
[0061] Link reaction:
[0062] Solution Ⅰ is a TaKaRa product. 8 μl double-enzyme-cut vector pET28a, 2 μl double-enzyme-cut PCR product, 10 μl solution Ⅰ, ligated overn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com