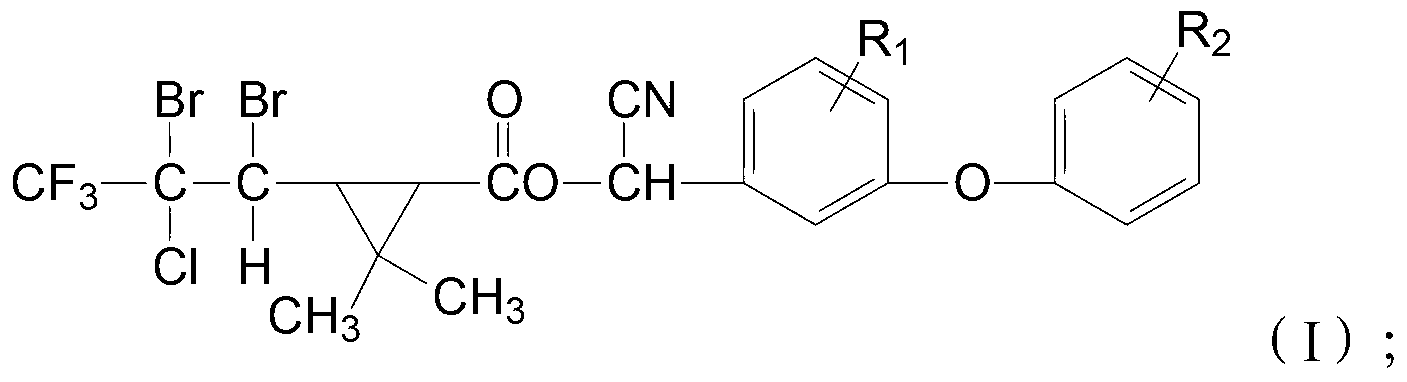
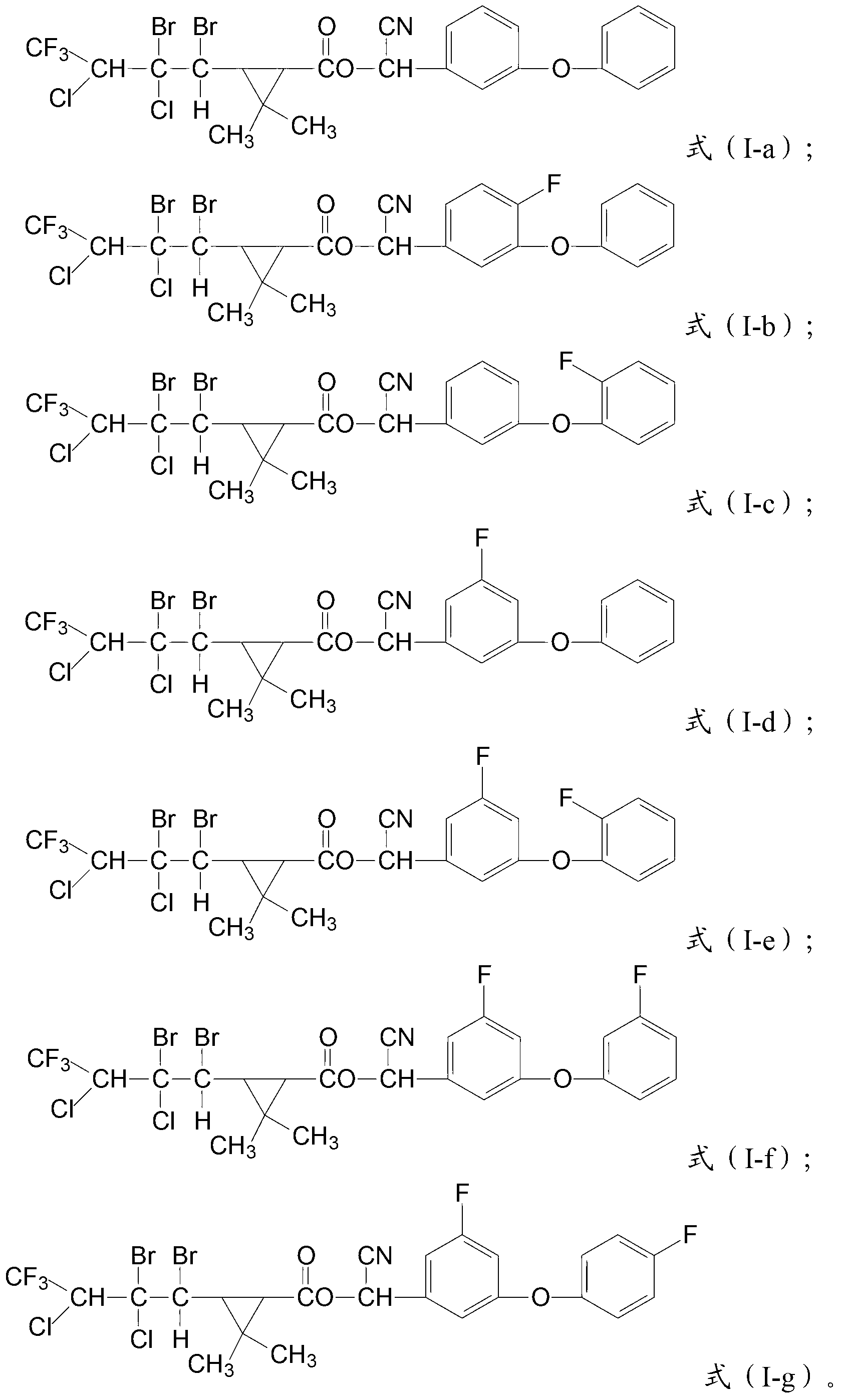
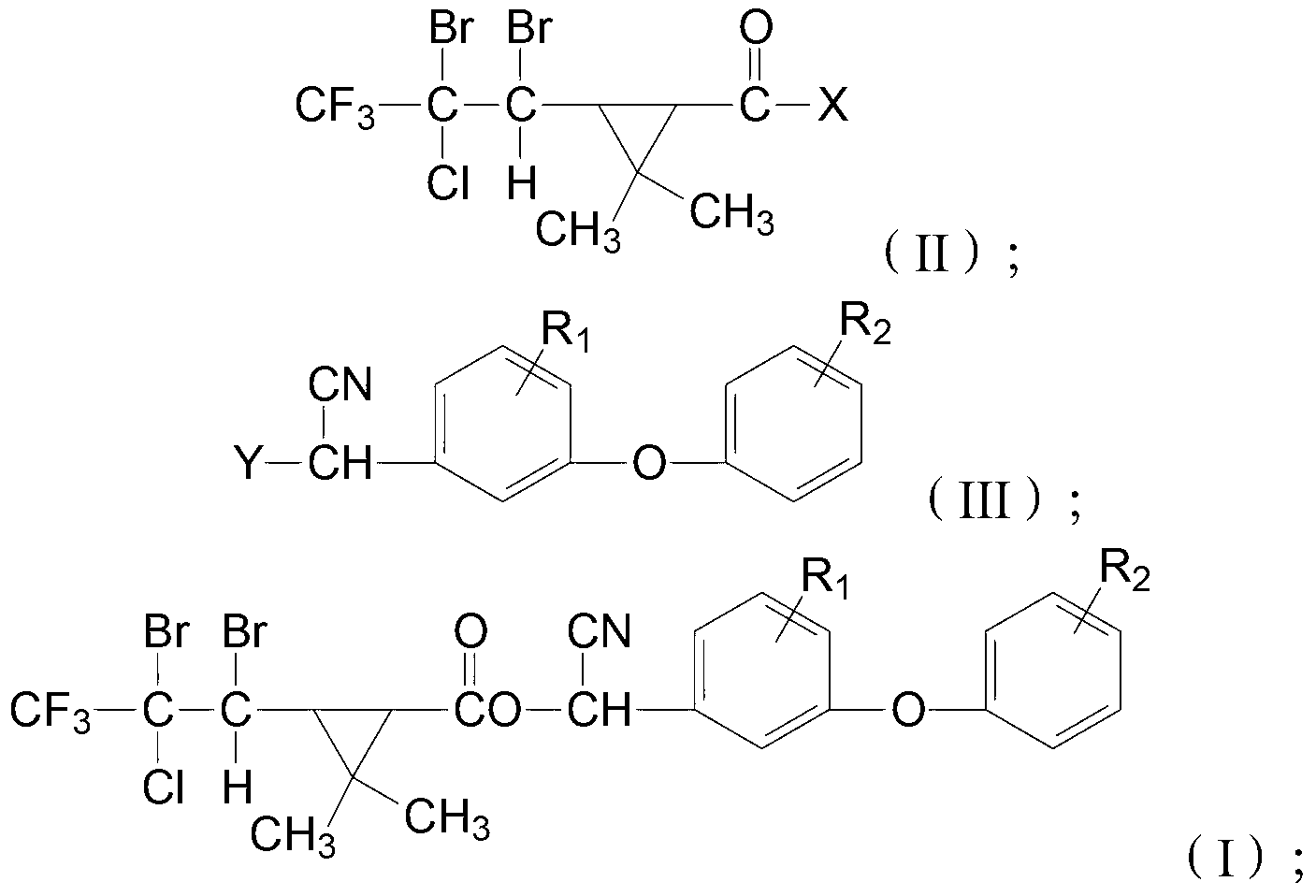
Cyclopropane carboxylic ester type compound, and preparation and application of same
A technology of tertiary amine compounds and compounds, applied in the field of pesticides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] In a reaction flask equipped with a thermometer and a stirrer, add 24.75g of 3-(2-chloro-3,3,3-trifluoro-1-propenyl)-2,2-dimethylcyclopropanecarboxylic acid and 200g of tetra Carbon chloride, after stirring evenly at room temperature, add dropwise a carbon tetrachloride solution containing bromine under 20°C, the carbon tetrachloride solution containing bromine is formed by dissolving 16.5g of bromine in 50g of carbon tetrachloride ; Then add 0.5g triethylamine, stir the reaction at room temperature for 8 hours, get 1-(1,2-dibromo-2-chloro-3,3,3-trifluoro-1-propenyl) after removing the solvent -2,2-Dimethylcyclopropanecarboxylic acid;
[0061] In a reaction flask equipped with a thermometer and a stirrer, add 32.84g of 1-(1,2-dibromo-2-chloro-3,3,3-trifluoro-1-propenyl)-2,2-dimethyl Cyclopropanecarboxylic acid, 150mL dichloromethane and 1mL dimethylformamide, add 10g thionyl chloride dropwise at room temperature, heat up to reflux, stir and react under reflux for 3 hou...
Embodiment 2
[0065] Put 26.6g of 3-(2-chloro-3,3,3-trifluoro-1-propenyl)-2,2-dimethylcyclopropaneyl chloride, 100g of tetrachloride Carbon and 0.3g triethylamine, below 20 ℃, add dropwise the carbon tetrachloride solution containing bromine, the carbon tetrachloride solution containing bromine is formed by dissolving 16.5g bromine in 50g carbon tetrachloride, After that, it was stirred at room temperature for 4 hours, and the solvent was removed to obtain the compound having the structure of formula (II-a), with a content of 96% and a yield of 95%.
[0066] In a reaction flask equipped with a thermometer and a stirrer, put 22g of 3-phenoxy-4-fluorobenzaldehyde, 100g of benzene, 8.2g of potassium cyanide, 30g of water and 1g of tetrabutylammonium bromide, stir evenly, and cool down to Below 15°C, add 43.8g of the above-prepared compound with the structure of formula (II-a) dropwise, after the dropwise addition, stir and react at room temperature for 10 hours, wash the obtained reactant with...
Embodiment 3
[0069] In a 1000mL reaction flask equipped with a thermometer and a stirrer, add 49g of crude oil of kaufthrin with a purity of 93%, 200g of carbon tetrachloride and 1g of triethylamine, and drop carbon tetrachloride containing bromine below 20°C Solution, the carbon tetrachloride solution containing bromine is formed by dissolving 16.5g bromine in 50g carbon tetrachloride, and the dropwise addition is completed in about 2 hours. Afterwards, the reaction was stirred at room temperature for 48 hours, and the compound with the structure of formula (I-a) was obtained after removing the solvent, with a content of 93% and a yield of 94%.
[0070] The compound having the structure of formula (I-a) was tested, and the result showed that it had the structure of formula (I-b).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com