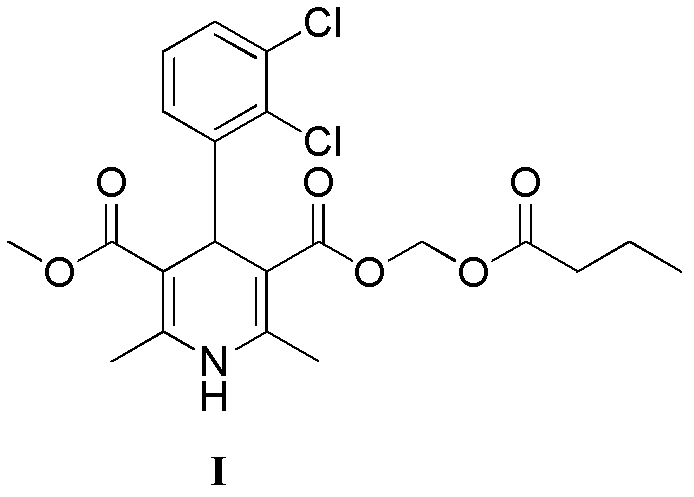
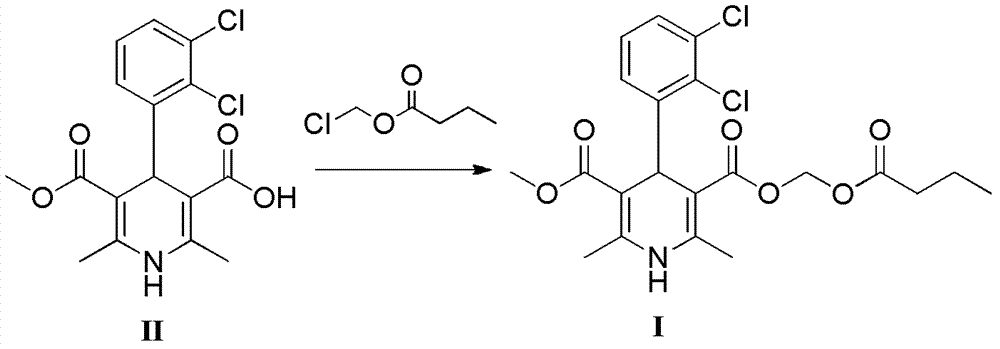
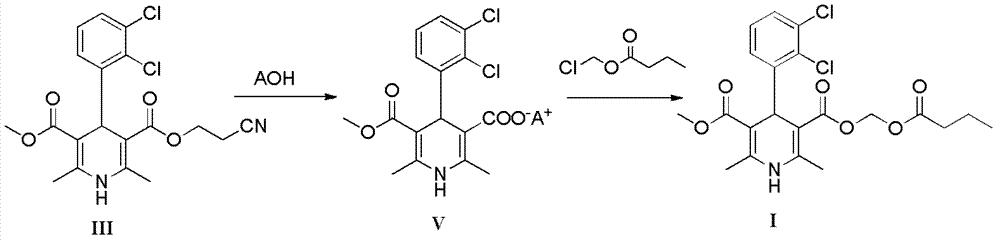
Preparation method of clevidipine butyrate
A technology of clevidipine butyrate and dichlorobenzaldehyde, which is applied in the direction of organic chemistry, can solve the problems of long reaction steps, high price, cumbersome operation, etc., and achieve the effects of low cost, low environmental pollution and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Preparation of (±)-4-(2′,3′-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-5-carboxylic acid methyl-3-carboxylic acid (II)
[0048] Method A:
[0049]Add 2,3-dichlorobenzaldehyde (1.75g, 10mmol), methyl acetoacetate (1.16g, 10mmol) and cyanoethyl 3-aminocrotonate (1.54g, 10mmol) successively in a round bottom flask, triethyl Amine (0.10g, 1mmol) and ethanol (50ml) were used as solvent, and reacted at 75°C for 8h. Cooling and cooling, suction filtration to obtain a solid, the resulting solid was recrystallized by adding ethanol (30ml), and suction filtration to obtain the compound (±)-3-(2-cyanoethyl)-5-methyl-4-(2',3'- Dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylic acid (III) (3.3 g, 8 mmol). Compound III (3.3g, 8mmol) was dissolved in ethanol (50ml), 8.8ml (8.8mmol) of 1M lithium hydroxide solution was added at 0°C, stirred for 2h, the solvent was evaporated, water (33ml) was added, and dilute Adjust the pH to 5-6 with hydrochloric acid (1M), an...
Embodiment 2
[0056] (±)-4-(2′,3′-dichlorophenyl)-2,6,-dimethyl-1,4-dihydropyridine-3,5-dicarboxylic acid methyl (butyryloxy Methyl) ester (I)
[0057] Method A:
[0058] Add (±)-4-(2′,3′-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-5-carboxylic acid methyl-3- Formic acid (3.1g, 8.7mmol), sodium bicarbonate (1.46g, 17.4mmol), potassium iodide (0.14g, 0.87mmol) and acetonitrile (30ml), then added chloromethyl n-butyrate (1.30g, 9.6mmol), Stirring and reflux reaction at 80°C for 6h. Cool to 50°C, heat filter, add isopropanol (10ml) and water (50ml) to the filtrate successively at 40°C, stir for 1 hour, then cool down to room temperature and stir overnight, a solid precipitates, filters to obtain a light yellow solid, filter cake Recrystallize with 20ml of isopropanol / water (2 / 1, volume ratio) to obtain 3.1g of solid with a yield of 77.5%.
[0059] mp.136.3~138.4℃; m / z: 478 (M+Na + ), 494 (M+K + ); 1 H NMR (DMSO, ppm): 9.03(s, 1H7.23-7.39(m, 3H), 5.67(dd, 2H, J=5.5Hz, J=5.38Hz), 5.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com