Preparation method of tetramisole hydrochloride
A technology of tetraimidazole hydrochloride and step 4 is applied in the field of preparation of tetraimidazole hydrochloride, which can solve the problems of complicated process and low yield, and achieve the effects of simplified operation steps, high purity and few impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
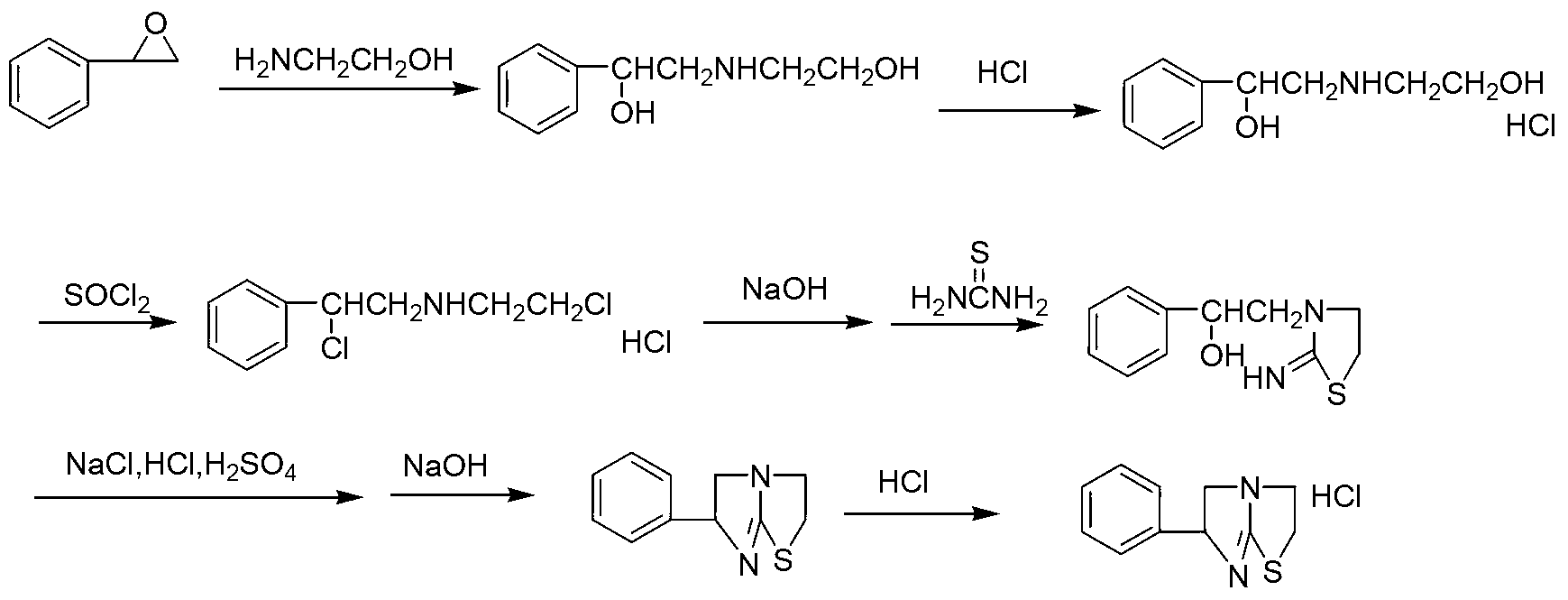
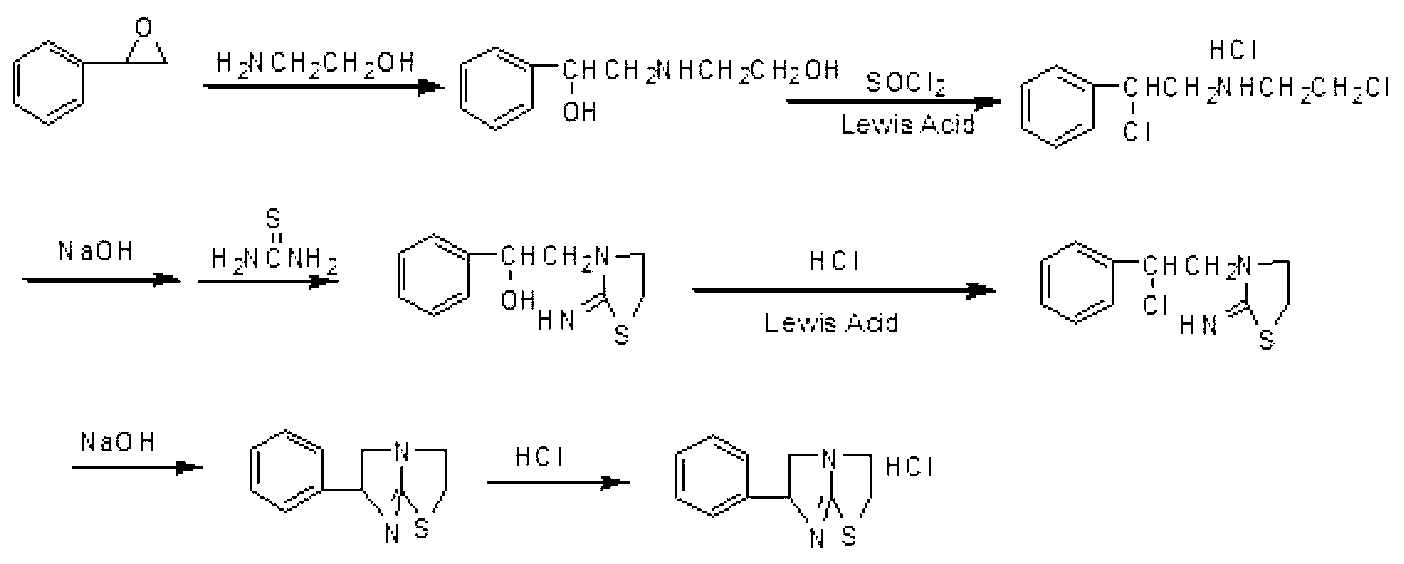
[0018] Specific embodiment one: the preparation method of tetramisole hydrochloride of the present embodiment realizes according to the following steps:
[0019] 1. Add styrene oxide and ethanolamine into the reaction flask at a molar ratio of 1: (4.5-5.5), heat to 40-80°C, stir for 2-4 hours, and filter to obtain the addition product;
[0020] 2. Add organic solvent and Lewis acid a to the addition product obtained in step 1, stir evenly, and add two Chlorothionyl chloride, then heated up to 40-60°C, reacted for 2-4 hours, and filtered to obtain the chlorinated product;
[0021] 3. Add deionized water to the chlorination product obtained in step 2, heat to 80-90°C, add NaOH solution dropwise, adjust the pH of the system to 2-2.3, and add concentrated HCl dropwise after reacting for 4-6 hours to adjust When the pH of the system reaches 1.5-3, then add thiourea, heat and reflux for 6-9 hours, and cool to 0°C to precipitate the cyclization product;
[0022] 4. Add the cyclizat...
specific Embodiment approach 2
[0027] Specific embodiment two: the difference between this embodiment and specific embodiment one is that the organic solvent in step two is benzene, toluene, xylene, dichloromethane or dichloroethane chloroform. Other steps and parameters are the same as those in Embodiment 1.
specific Embodiment approach 3
[0028] Specific embodiment three: the difference between this embodiment and specific embodiment one or two is that step two Lewis acid a is iron trichloride, titanium tetrachloride, zinc chloride, magnesium dichloride, lithium chloride or phosphorus trichloride. Other steps and parameters are the same as those in Embodiment 1 or Embodiment 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com