Method for preparing photosensitive graft polymer containing double-azobenzene
A technology of grafting polymer and bisazobenzene is applied in the field of preparation of photosensitive grafting polymer, which can solve the problems of single structure and low molecular weight of polymer, and achieve the effect of convenient design.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
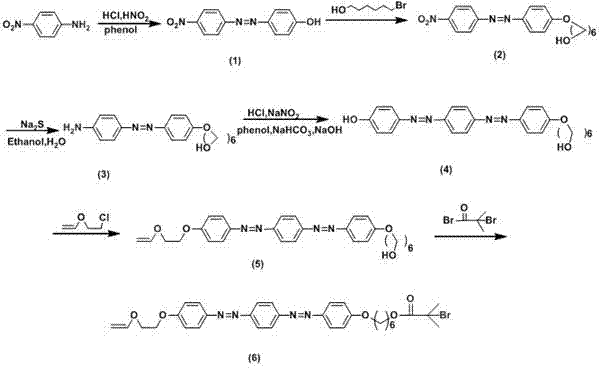
[0034] A preparation method of a photosensitive graft polymer containing bisazobenzene, comprising the following steps:
[0035] Step 1) Use p-nitroaniline and phenol as raw materials to synthesize p-nitroazophenol, and carry out sequential chain reaction of phenolic hydroxyl group to alcoholic hydroxyl group; nitro group is reduced to amino group; and phenol azotization to introduce disazo group group; phenolic hydroxyl group and 2-chloroethyl vinyl ether carry out electrophilic substitution reaction to introduce vinyl ether group as monomer for living cationic polymerization; alcoholic hydroxyl group and α -Bromoisobutyryl bromide introduced by esterification α -Bromoisobutyl group as the initiator of AGET ATRP; the final compound obtained is 4-(4-(4-(4-(2-vinyloxy)ethoxy)azophenyl)azophenyl )phenoxy- α- Hexyl bromoisobutyrate (VEBiB);
[0036] Step 2) Using toluene as solvent, the concentration of each component is: monomer (1) 0.76 mol / L, monomer (2) 0.02-0.04 mol / L, Le...
Embodiment 1
[0052] Embodiment one: the synthesis of active cationic monomer AGET ATRP initiator compound VEBiB ( figure 1 )
[0053] Add p-nitroaniline (6.9 g, 50.0 mmol), 30 mL of deionized water and 15 mL of concentrated hydrochloric acid and stir in an ice-water bath to dissolve. o Slowly add sodium nitrite solution (3.9 g, 10 mL) dropwise at ℃, react for 40 min to make diazo component, phenol (8.0 g, 85.0 mmol), NaOH (4.0 g, 100.0 mmol), NaHCO 3 (4.2 g, 50.0 mmol) and 250 mL deionized water were stirred in an ice-water bath to form a coupling solution, and the pH was kept at 8~10, and the temperature was 0~5 o During C, the diazo component was added dropwise to the coupling solution, and the final mixed solution was reacted for 2 h, filtered, dried in vacuo, and purified by recrystallization from absolute ethanol to obtain a dark red compound (1).
[0054] DMF was used as a solvent to dissolve compound (1) (6.1 g, 25.0 mmol) in stirring, then add 6-bromohexanol (5.4 g, 30.0 mmol), K...
Embodiment 2
[0059] Embodiment two: the active cationic copolymerization of IBVE and VEBiB
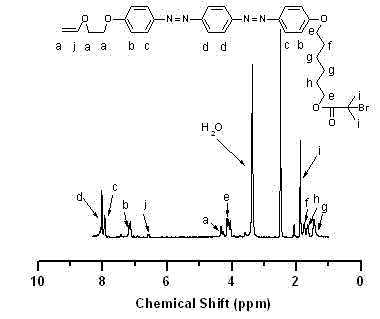
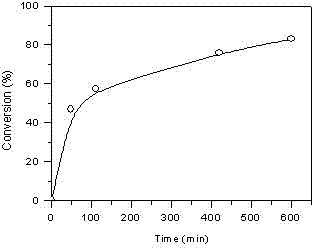
[0060] In the glove box, take monomer VEBiB (0.02 mol / L, 0.135 g), monomer IBVE (0.76 mol / L, 1 mL), solvent toluene, add alkali ethyl acetate (1 mol / L, 0.98 mL), Lewis acid catalyst Et 1.5 AlCl 1.5 (20 mmol / L, 0.5 mL), cationic initiator IBEA (4 mmol / L, 7 μL) were put into a 30 mL polymerization bottle and placed at 0 o C reacts according to the predetermined time (1~24 h). At the end of the polymerization, a terminator is added, and methanol containing a small amount of ammonia water is used to terminate the reaction. The mixture is taken out from the glove box and precipitated with methanol, filtered, and dried to obtain the polymer PIBVE- co -PVEBiB, GPC and 1 H NMR characterization.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com