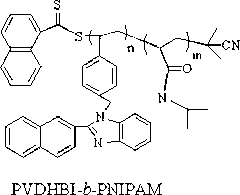
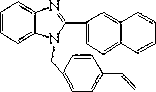
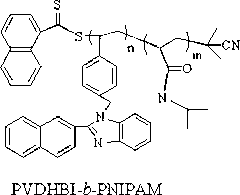
Synthetic method of amphiphilic block polymer with near infrared fluorescence characteristic
A technology of amphiphilic block and synthesis method, which is applied in the field of synthesis of amphiphilic block polymers, and can solve problems such as few types, light, heat, unstable chemical properties, and difficult synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 1. There are two main steps in the synthesis of monomeric VDHBI:
[0044] (1) Combine o-phenylenediamine with β -After the naphthoic acid is mixed evenly, put it into a 10mL ampule bottle, and then add polyphosphoric acid. In an oil bath at about 180°C, vacuumize and react for about 30 minutes. Cool naturally after the reaction, add DMSO to dissolve. The solution was poured into deionized water, stirred, and a precipitate appeared. The filter cake was obtained by suction filtration, washed with water, filtered by suction, and recrystallized with absolute ethanol to obtain the pure product.
[0045] (2) Add DMF in a three-neck round bottom flask, intermediate α -Benzimidazolyl naphthalene, K 2 CO 3 (Grind finely), stir for about 20 minutes until completely dissolved. Add p-chloromethylstyrene (VBC), heat to 38°C-42°C, and react at this temperature for more than 48h. Spot plate control reaction end point, column chromatography purification.
[0046] 2. Synthesis ...
Embodiment 2
[0053] Embodiment two: block polymer PNIPAM- b - Self-assembly performance test of PVDHBI
[0054] In the block polymer PNIPAM- b - In the PVDHBI structure, the VDHBI segment is hydrophobic, while the NIPAM segment is hydrophilic. from figure 1 , figure 2 with image 3 It can be seen from the figure that in the THF solution of the block polymer, as the volume of the added water gradually increases, the fluorescence intensity of the polymer solution also gradually increases, which is because the polymer in the mixed solution of water and THF occurs Self-assembly. The fluorescence intensity reaches the maximum when the volume of water is 70% of the volume of THF. At the same time, it can be seen from the DLS curve that ( image 3 ), the particle size of the polymer increases with the increase of the amount of water added, which confirms that the polymer aggregates in the solution.
Embodiment 3
[0055] Embodiment three: block polymer PNIPAM- b - pH sensitivity test of PVDHBI
[0056] Due to the nitrogen atom on the benzimidazole, when the pH of the polymer THF solution was changed, the fluorescence intensity changed obviously. from Figure 4 with Figure 5 It can be seen that the fluorescence intensity of the block polymer solution changes significantly with the increase or decrease of pH, and can change repeatedly from strong to weak or from weak to strong. When the solution changed from neutral to alkaline, the fluorescence of the polymer solution did not change much. The solution is acidic, that is, when the pH value gradually decreases, the nitrogen atom on the benzimidazole is gradually protonated, resulting in a gradual red shift of the fluorescence emission peak of the polymer solution, and a gradual increase in fluorescence intensity. When the pH value is around 3, the fluorescence intensity reaches the maximum.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com